SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs
- PMID: 34915880
- PMCID: PMC8680308
- DOI: 10.1186/s12933-021-01430-3
SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs
Abstract
Background: It has been suggested that sodium-glucose cotransporter 2 (SGLT-2) inhibitors reduce the cardiorenal risk in patients with type 2 diabetes (T2D). The purpose of this study is to provide an update of all large cardiovascular outcome trials (CVOTs) with SGLT-2 inhibitors to assess their cardiorenal efficacy in patients with and without T2D.
Methods: An electronic search up to 30 September 2021 was conducted in PubMed, EMBASE, the Cochrane Database of Systematic Reviews, and ClinicalTrials.gov. to determine eligible trials. We included CVOTs comparing any SGLT-2 inhibitor with placebo, reporting desired cardiovascular or renal outcomes and with a follow-up duration of at least 6 months.
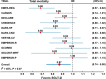
Results: Eleven CVOTs, with data from five SGLT-2 inhibitors (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin and sotagliflozin) and 77,541 participants, were included. In the overall analysis, the risk of the composite CV mortality or hospitalization for heart failure (HF) was reduced by 23% (HR = 0.77, 95% CI 0.73-0.82, P < 0.001) compared with placebo, with not significant heterogeneity (I2 = 26%, P = 0.20), and irrespective of the presence of T2D (P for interaction = 0.81) and age (> 65 vs ≤ 65 years, P for interaction = 0.78). The risk of CV mortality, total mortality and hospitalization for HF was significantly reduced by 16%, 13%, and 32%, respectively; similarly, the risk of the composite renal outcome was reduced by 35% (HR = 0.65, 95% CI 0.56-0.75), with moderate heterogeneity (I2 = 32%). In the analysis of 6 CVOTs reporting the data, the risk of major cardiovascular events (MACE) was reduced by 12%, with low heterogeneity (I2 = 21.2%, P = 0.19) and irrespective of the presence of established CV disease at baseline (P for interaction = 0.46).
Conclusions: Therapy with SGLT-2 inhibitors in patients with cardiometabolic and renal diseases results in a sustained to moderate reduction of the composite CV death or hospitalization for HF, robust reduction of HF and renal outcomes, moderate reduction of CV mortality, total mortality and MACE.
Keywords: Cardiorenal outcomes; Cardiovascular outcome trials; SGLT-2 inhibitors; Type 2 diabetes.
© 2021. The Author(s).
Conflict of interest statement
DG received honoraria for speaking at meetings from Novartis, Sanofi, Lilly, MundiPharma, Astrazeneca, and NovoNordisk. MIM received honoraria for speaking at meetings from Astrazeneca, NovoNordisk, Bruno, Mundipharma, Sanofi. KE received honoraria for speaking at meetings from Novartis, Sanofi‐Aventis, Lilly, AstraZeneca, MundiPharma, Boehringer Ingelheim, and NovoNordisk. No other potential competing interest relevant to this article was reported.
Figures






References
-
- Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring: Food and Drug Administration; 2008. http://www.fda.gov/downloads/Drugs/Guidances/ucm071627.pdf. Accessed 24 Aug 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

