Loeys-Dietz and Shprintzen-Goldberg syndromes: analysis of TGF-β-opathies with craniofacial manifestations using an innovative multimodality method
- PMID: 34916229
- PMCID: PMC9554024
- DOI: 10.1136/jmedgenet-2021-107695
Loeys-Dietz and Shprintzen-Goldberg syndromes: analysis of TGF-β-opathies with craniofacial manifestations using an innovative multimodality method
Abstract
Background: Elevated transforming growth factor-beta (TGF-β) signalling has been implicated in the pathogenesis of Loeys-Dietz syndrome (LDS) and Shprintzen-Goldberg syndrome (SGS). In this study, we provide a qualitative and quantitative analysis of the craniofacial and functional features among the LDS subtypes and SGS.
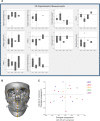
Methods: We explore the variability within and across a cohort of 44 patients through deep clinical phenotyping, three-dimensional (3D) facial photo surface analysis, cephalometric and geometric morphometric analyses of cone-beam CT scans.
Results: The most common craniofacial features detected in this cohort include mandibular retrognathism (84%), flat midface projection (84%), abnormal eye shape (73%), low-set ears (73%), abnormal nose (66%) and lip shape (64%), hypertelorism (41%) and a relatively high prevalence of nystagmus/strabismus (43%), temporomandibular joint disorders (38%) and obstructive sleep apnoea (23%). 3D cephalometric analysis demonstrated an increased cranial base angle with shortened anterior cranial base and underdevelopment of the maxilla and mandible, with evidence of a reduced pharyngeal airway in 55% of those analysed. Geometric morphometric analysis confirmed that the greatest craniofacial shape variation was among patients with LDS type 2, with distinct clustering of patients with SGS.
Conclusions: This comprehensive phenotypic approach identifies developmental abnormalities that segregate to mutation variants along the TGF-β signalling pathway, with a particularly severe phenotype associated with TGFBR2 and SKI mutations. Multimodality assessment of craniofacial anomalies objectively reveals the impact of mutations of the TGF-β pathway with perturbations associated with the cranium and cranial base with severe downstream effects on the orbit, maxilla and mandible with the resultant clinical phenotypes.
Keywords: aneurysm; cardiovascular abnormalities; genetic heterogeneity; human genetics; phenotype.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or Tgfbr2. Nat Genet 2005;37:275–81. 10.1038/ng1511 - DOI - PubMed
-
- Bertoli-Avella AM, Gillis E, Morisaki H, Verhagen JMA, de Graaf BM, van de Beek G, Gallo E, Kruithof BPT, Venselaar H, Myers LA, Laga S, Doyle AJ, Oswald G, van Cappellen GWA, Yamanaka I, van der Helm RM, Beverloo B, de Klein A, Pardo L, Lammens M, Evers C, Devriendt K, Dumoulein M, Timmermans J, Bruggenwirth HT, Verheijen F, Rodrigus I, Baynam G, Kempers M, Saenen J, Van Craenenbroeck EM, Minatoya K, Matsukawa R, Tsukube T, Kubo N, Hofstra R, Goumans MJ, Bekkers JA, Roos-Hesselink JW, van de Laar IMBH, Dietz HC, Van Laer L, Morisaki T, Wessels MW, Loeys BL. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol 2015;65:1324–36. 10.1016/j.jacc.2015.01.040 - DOI - PMC - PubMed
-
- Doyle AJ, Doyle JJ, Bessling SL, Maragh S, Lindsay ME, Schepers D, Gillis E, Mortier G, Homfray T, Sauls K, Norris RA, Huso ND, Leahy D, Mohr DW, Caulfield MJ, Scott AF, Destrée A, Hennekam RC, Arn PH, Curry CJ, Van Laer L, McCallion AS, Loeys BL, Dietz HC. Mutations in the TGF-β repressor Ski cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet 2012;44:1249–54. 10.1038/ng.2421 - DOI - PMC - PubMed
-
- Carmignac V, Thevenon J, Adès L, Callewaert B, Julia S, Thauvin-Robinet C, Gueneau L, Courcet J-B, Lopez E, Holman K, Renard M, Plauchu H, Plessis G, De Backer J, Child A, Arno G, Duplomb L, Callier P, Aral B, Vabres P, Gigot N, Arbustini E, Grasso M, Robinson PN, Goizet C, Baumann C, Di Rocco M, Sanchez Del Pozo J, Huet F, Jondeau G, Collod-Beroud G, Beroud C, Amiel J, Cormier-Daire V, Rivière J-B, Boileau C, De Paepe A, Faivre L. In-Frame mutations in exon 1 of Ski cause dominant Shprintzen-Goldberg syndrome. Am J Hum Genet 2012;91:950–7. 10.1016/j.ajhg.2012.10.002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
