Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial
- PMID: 34916266
- PMCID: PMC8678498
- DOI: 10.1183/13993003.02518-2021
Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial
Abstract
Background: Low-dose dexamethasone demonstrated clinical improvement in patients with coronavirus disease 2019 (COVID-19) needing oxygen therapy; however, evidence on the efficacy of high-dose dexamethasone is limited.
Methods: We performed a randomised, open-label, controlled trial involving hospitalised patients with confirmed COVID-19 pneumonia needing oxygen therapy. Patients were randomly assigned in a 1:1 ratio to receive low-dose dexamethasone (6 mg once daily for 10 days) or high-dose dexamethasone (20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days). The primary outcome was clinical worsening within 11 days since randomisation. Secondary outcomes included 28-day mortality, time to recovery and clinical status at day 5, 11, 14 and 28 on an ordinal scale ranging from 1 (discharged) to 7 (death).
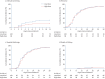
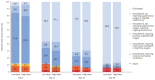
Results: A total of 200 patients (mean±sd age 64±14 years; 62% male) were enrolled. 32 (31.4%) out of 102 patients enrolled in the low-dose group and 16 (16.3%) out of 98 in the high-dose group showed clinical worsening within 11 days since randomisation (rate ratio 0.427, 95% CI 0.216-0.842; p=0.014). The 28-day mortality was 5.9% in the low-dose group and 6.1% in the high-dose group (p=0.844). There was no significant difference in time to recovery, and in the seven-point ordinal scale at days 5, 11, 14 and 28.
Conclusions: Among hospitalised COVID-19 patients needing oxygen therapy, high dose of dexamethasone reduced clinical worsening within 11 days after randomisation, compared with low dose.
Trial registration: ClinicalTrials.gov NCT04726098.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: The authors declare the absence of conflict of interests.
Figures
References
-
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group , Sterne JAC, Murthy S, et al. . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi:10.1001/jama.2020.17023 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
