Real-life ruxolitinib experience in intermediate-risk myelofibrosis
- PMID: 34916339
- PMCID: PMC8721444
- DOI: 10.5045/br.2021.2021101
Real-life ruxolitinib experience in intermediate-risk myelofibrosis
Abstract
Background: In this retrospective cohort of patients with primary, post-polycythemia vera, or post-essential thrombocythemia myelofibrosis, 57 patients with MF who received ruxolitinib for MF-related symptoms or symptomatic splenomegaly were evaluated.
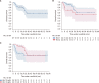
Methods: The median age of the patients in this cohort was approximately 58 years. Of these, there were 33 patients (57.9%) in INT-1, 23 patients (40.4%) in INT-2, and 1 patient (1.8%) at high risk. Overall, spleen size reduction of at least 35% (spleen response) was achieved in 56.6% and 63.3% of all cohort and INT-1 risk at any time, respectively.
Results: Symptom response and clinical improvement were observed in 21.7% and 60.7% of patients, respectively. Anemia and thrombocytopenia were prevalent, but manageable. About 73.7% of patients continued treatment during a median follow-up of 22 months. Two-year OS probability was approximately 84.5% (95% CI, 63.1‒94.0%) and 62.3% (95% CI, 37.5‒79.6%) for the intermediate-1 and -2 risk groups, respectively.
Conclusion: Real-life experience in a community-based hospital confirms the efficacy and safety profile of ruxolitinib in intermediate-risk myelofibrosis. Treatment discontinuation rates were lower than those in clinical trials.
Keywords: Primary myelofibrosis; Ruxolitinib; Spleen response.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–8. doi: 10.1182/blood-2009-09-245837. - DOI - PubMed
-
- Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7. doi: 10.1200/JCO.2010.32.2446. - DOI - PubMed
LinkOut - more resources
Full Text Sources