Effects of electroacupuncture on the functionality of NG2-expressing cells in perilesional brain tissue of mice following ischemic stroke
- PMID: 34916441
- PMCID: PMC8771106
- DOI: 10.4103/1673-5374.330611
Effects of electroacupuncture on the functionality of NG2-expressing cells in perilesional brain tissue of mice following ischemic stroke
Abstract
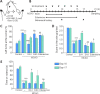
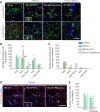
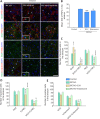
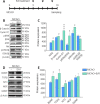
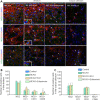
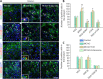
Neural/glial antigen 2 (NG2)-expressing cells has multipotent stem cell activity under cerebral ischemia. Our study examined the effects of electroacupuncture (EA) therapy (2 Hz, 1 or 3 mA, 20 minutes) at the Sishencong acupoint on motor function after ischemic insult in the brain by investigating the rehabilitative potential of NG2-derived cells in a mouse model of ischemic stroke. EA stimulation alleviated motor deficits caused by ischemic stroke, and 1 mA EA stimulation was more efficacious than 3 mA EA stimulation or positive control treatment with edaravone, a free radical scavenger. The properties of NG2-expressing cells were altered with 1 mA EA stimulation, enhancing their survival in perilesional brain tissue via reduction of tumor necrosis factor alpha expression. EA stimulation robustly activated signaling pathways related to proliferation and survival of NG2-expressing cells and increased the expression of neurotrophic factors such as brain-derived neurotrophic factor, tumor growth factor beta, and neurotrophin 3. In the perilesional striatum, EA stimulation greatly increased the number of NG2-expressing cells double-positive for oligodendrocyte, endothelial cell, and microglia/macrophage markers (CC1, CD31, and CD68). EA therapy also greatly activated brain-derived neurotrophic factor/tropomyosin receptor kinase B and glycogen synthase kinase 3 beta signaling. Our results indicate that EA therapy may prevent functional loss at the perilesional site by enhancing survival and differentiation of NG2-expressing cells via the activation of brain-derived neurotrophic factor -induced signaling, subsequently ameliorating motor dysfunction. The animal experiments were approved by the Animal Ethics Committee of Pusan National University (approval Nos. PNU2019-2199 and PNU2019-2884) on April 8, 2019 and June 19, 2019.
Keywords: brain-derived neurotrophic factor; differentiation; electroacupuncture; motor function; neural/glial antigen 2; perilesional striatum; stroke; survival.
Conflict of interest statement
None
Figures

Similar articles
-
Therapeutic Potential of a Combination of Electroacupuncture and TrkB-Expressing Mesenchymal Stem Cells for Ischemic Stroke.Mol Neurobiol. 2019 Jan;56(1):157-173. doi: 10.1007/s12035-018-1067-z. Epub 2018 Apr 22. Mol Neurobiol. 2019. PMID: 29682700
-
Electroacupuncture Therapy Ameliorates Motor Dysfunction via Brain-Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor in a Mouse Model of Parkinson's Disease.J Gerontol A Biol Sci Med Sci. 2020 Mar 9;75(4):712-721. doi: 10.1093/gerona/glz256. J Gerontol A Biol Sci Med Sci. 2020. PMID: 31644786
-
Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia.PLoS One. 2014 Feb 24;9(2):e90000. doi: 10.1371/journal.pone.0090000. eCollection 2014. PLoS One. 2014. PMID: 24587178 Free PMC article.
-
NG2-glia cell proliferation and differentiation by glial growth factor 2 (GGF2), a strategy to promote functional recovery after ischemic stroke.Biochem Pharmacol. 2020 Jan;171:113720. doi: 10.1016/j.bcp.2019.113720. Epub 2019 Nov 18. Biochem Pharmacol. 2020. PMID: 31751533 Review.
-
Roles of NG2-glia in ischemic stroke.CNS Neurosci Ther. 2017 Jul;23(7):547-553. doi: 10.1111/cns.12690. Epub 2017 Mar 19. CNS Neurosci Ther. 2017. PMID: 28317272 Free PMC article. Review.
Cited by
-
Effect of Combination Electroacupuncture and Tenuigenin on the Migration and Differentiation of Mesenchymal Stem Cells following Ischemic Stroke.J Pharmacopuncture. 2023 Dec 31;26(4):357-365. doi: 10.3831/KPI.2023.26.4.357. J Pharmacopuncture. 2023. PMID: 38162470 Free PMC article.
-
Acupuncture for stroke: A bibliometric analysis of global research from 2000 to 2022.Heliyon. 2024 Jun 28;10(13):e33827. doi: 10.1016/j.heliyon.2024.e33827. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39050433 Free PMC article.
-
A novel phenotype of B cells associated with enhanced phagocytic capability and chemotactic function after ischemic stroke.Neural Regen Res. 2023 Nov;18(11):2413-2423. doi: 10.4103/1673-5374.371365. Neural Regen Res. 2023. PMID: 37282471 Free PMC article.
-
Electroacupuncture for the treatment of ischemic stroke: A preclinical meta-analysis and systematic review.Neural Regen Res. 2026 Mar 1;21(3):1191-1210. doi: 10.4103/NRR.NRR-D-24-01030. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885673 Free PMC article.
-
Acupuncture and Acupoints for Managing Pediatric Cerebral Palsy: A Meta-Analysis of Randomized Controlled Trials.Healthcare (Basel). 2024 Sep 5;12(17):1780. doi: 10.3390/healthcare12171780. Healthcare (Basel). 2024. PMID: 39273805 Free PMC article. Review.
References
-
- Ahn SM, Jung DH, Lee HJ, Pak ME, Jung YJ, Shin YI, Shin HK, Choi BT. Contralesional application of transcranial direct current stimulation on functional improvement in ischemic stroke mice. Stroke. 2020;51:2208–2218. - PubMed
-
- Ahn SM, Kim YR, Shin YI, Ha KT, Lee SY, Shin HK, Choi BT. Therapeutic potential of a combination of electroacupuncture and TrkB-expressing mesenchymal stem cells for ischemic stroke. Mol Neurobiol. 2019;56:157–173. - PubMed
-
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

