Role of neuropeptide neuromedin U in the nucleus accumbens shell in cocaine self-administration in male rats
- PMID: 34916591
- PMCID: PMC9485260
- DOI: 10.1038/s41386-021-01234-9
Role of neuropeptide neuromedin U in the nucleus accumbens shell in cocaine self-administration in male rats
Abstract
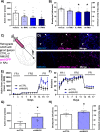
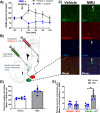
The nucleus accumbens shell (NAcSh) and its afferent and efferent neuronal projections control key aspects of motivation for cocaine. A recently described regulator of γ-aminobutyric acid (GABA) projections from the dorsal raphe nucleus (DRN) to the NAcSh (DRN → NAcSh) is the neuropeptide neuromedin U (NMU). Here, we find that systemic administration of NMU decreases breakpoint for cocaine on a progressive ratio schedule of reinforcement in male rats. Employing a retrograde adeno-associated virus (AAV), we found that RNAi-mediated knockdown of the NMU receptor 2 (NMUR2) in afferent DRN projections to the NAcSh increases the breakpoint for cocaine. Our previous studies demonstrated that NMU regulates GABA release in the NAcSh, and our current investigation found that systemic NMU administration suppresses cocaine-evoked GABA release in the NAcSh and increases phosphorylated c-Fos expression in neurons projecting from the NAcSh to the ventral pallidum (VP). To further probe the impact of NMU/NMUR2 on neuroanatomical pathways regulating motivation for cocaine, we employed multi-viral transsynaptic studies. Using a combination of rabies virus and retrograde AAV helper virus, we mapped the impact of NMU across three distinct brain regions simultaneously and found a direct connection of GABAergic DRN neurons to the NAcSh → VP pathway. Together, these data reveal that NMU/NMUR2 modulates a direct connection within the GABAergic DRN → NAcSh → VP circuit that diminishes breakpoints for cocaine. These findings importantly advance our understanding of the neurochemical underpinnings of pathway-specific regulation of neurocircuitry that may regulate cocaine self-administration, providing a unique therapeutic perspective.
© 2021. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
Dr. Cunningham has current research funding from VidaLibreBio, Inc., for research unrelated to this study. Additional authors declare no competing interests.
Figures


Comment in
-
Neuromedin U: a neuropeptide modulator of GABA transmission contributes to cocaine seeking.Neuropsychopharmacology. 2022 Oct;47(11):1873-1874. doi: 10.1038/s41386-021-01253-6. Epub 2021 Dec 23. Neuropsychopharmacology. 2022. PMID: 34949771 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

