Profiling Biomarkers in HIV Glomerular Disease - Potential for the Non-Invasive Diagnosis of HIVAN?
- PMID: 34916827
- PMCID: PMC8668162
- DOI: 10.2147/IJNRD.S331484
Profiling Biomarkers in HIV Glomerular Disease - Potential for the Non-Invasive Diagnosis of HIVAN?
Abstract
Background: There is a wide spectrum of kidney pathology in human immunodeficiency virus (HIV) infection, affecting all structures of the kidney. The histology of HIV chronic kidney disease (CKD) is diverse, ranging from HIV-associated nephropathy (HIVAN) to focal glomerulosclerosis (FSGS), HIV-immune complex disease (HIV-ICD), other glomerulopathies and tubulo-interstitial nephritis. Definitive diagnosis is by kidney biopsy, an invasive procedure. However, serum and urinary biomarkers may be useful in predicting the histological diagnosis of HIVAN.
Purpose: We wished to determine the utility of serum and urinary biomarkers in predicting the histological diagnosis of HIVAN.
Patients and methods: We measured neutrophil gelatinase-associated lipocalin (NGAL), cystatin C, transforming growth factor (TGF)-β isoforms and bone morphogenetic protein (BMP)-7 in the serum and urine in patients with different histological forms of HIV glomerular disease.
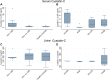
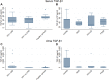
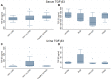
Results: In HIVAN, we demonstrated increased levels of serum cystatin C and increased levels of serum and urinary NGAL. Urinary TGF-β1 and TGF-β2 levels were elevated in HIV-positive patients with CKD but were not significantly different in the different HIV histologies, while urinary BMP-7 levels were elevated in minimal change disease.
Conclusion: This study confirmed the presence of increased serum and urinary biomarkers of tubular injury in patients with HIVAN, and increased urinary biomarkers of fibrosis in HIV CKD, and may indicate their value as a non-invasive diagnostic tool for the diagnosis of HIVAN.
Keywords: HIV chronic kidney disease; NGAL; bone morphogenetic protein (BMP)-7; cystatin C; neutrophil gelatinase-associated lipocalin; transforming growth factor (TGF)-β isoforms.
© 2021 Naicker et al.
Conflict of interest statement
Professor Saraladevi Naicker report grants from Medical Research Council of South Africa (MRC), during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures



References
-
- Fabian J, Naicker S, Venter WDF, et al. Urinary screening abnormalities in antiretroviral-naïve HIV-infected outpatients and implications for management- a single centre study in South Africa. Ethn Dis. 2009;S1:80–85. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

