Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies
- PMID: 34917509
- PMCID: PMC8669727
- DOI: 10.3389/fonc.2021.782766
Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies
Abstract
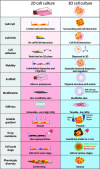
Over 90% of potential anti-cancer drug candidates results in translational failures in clinical trials. The main reason for this failure can be attributed to the non-accurate pre-clinical models that are being currently used for drug development and in personalised therapies. To ensure that the assessment of drug efficacy and their mechanism of action have clinical translatability, the complexity of the tumor microenvironment needs to be properly modelled. 3D culture models are emerging as a powerful research tool that recapitulates in vivo characteristics. Technological advancements in this field show promising application in improving drug discovery, pre-clinical validation, and precision medicine. In this review, we discuss the significance of the tumor microenvironment and its impact on therapy success, the current developments of 3D culture, and the opportunities that advancements that in vitro technologies can provide to improve cancer therapeutics.
Keywords: 3D bioprinting; 3D culture systems; drug resistance prevention; extracellular matrix; microfluidics; personalised medicine; tumor microenvironment.
Copyright © 2021 Law, Rodriguez de la Fuente, Grundy, Fang, Valdes-Mora and Gallego-Ortega.
Conflict of interest statement
TG is employed by Inventia Life Science Pty Ltd as stated in affiliations. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

