Ripening of two-dimensional colloidal CdSe nanocrystals into zero-dimensional nanodots
- PMID: 34917893
- PMCID: PMC8646172
- DOI: 10.1016/j.isci.2021.103457
Ripening of two-dimensional colloidal CdSe nanocrystals into zero-dimensional nanodots
Abstract
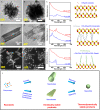
Understanding the ripening of two-dimensional (2D) colloidal nanocrystals (NCs) is important for the controllable synthesis of NCs with desired morphology and properties. In this study, we systematically investigate the ripening behavior of the 2D CdSe NCs in the presence of a short-chain acetate ligand and a long-chain oleate ligand. We find that a low acetate/oleate ratio, a low Cd/Se ratio, and a low monomer concentration help in the ripening of the 2D NCs to form 0D NCs. Moreover, a porous nanosheet intermediate is observed when there is a high Cd/Se ratio, whereas in the case of a low Cd/Se ratio, the ripening starts from the edge of the nanosheets, resulting in a saw-like nanosheet intermediate. These findings provide necessary insights into the growth and ripening of 2D CdSe NCs that allow for the controlled synthesis of 0D and 2D CdSe NCs.
Keywords: Colloids; Nanomaterials; Nanoparticles.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Bertrand G.H., Polovitsyn A., Christodoulou S., Khan A.H., Moreels I. Shape control of zincblende CdSe nanoplatelets. Chem. Commun. 2016;52:11975–11978. - PubMed
-
- Bouet C., Tessier M.D., Ithurria S., Mahler B., Nadal B., Dubertret B. Flat colloidal semiconductor nanoplatelets. Chem. Mater. 2013;25:1262–1271.
-
- Chen Z., Nadal B., Mahler B., Aubin H., Dubertret B. Quasi-2D colloidal semiconductor nanoplatelets for narrow electroluminescence. Adv. Funct. Mater. 2014;24:295–302.
LinkOut - more resources
Full Text Sources

