Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: An observational study
- PMID: 34918001
- PMCID: PMC8642706
- DOI: 10.1016/j.lanepe.2021.100235
Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: An observational study
Abstract
Background: High-risk human papillomavirus (hrHPV) testing on self-collected samples has potential as a primary screening tool in cervical screening, but real-world evidence on its accuracy in hrHPV-based screening programmes is lacking.
Methods: In the Netherlands, women aged 30-60 years invited for cervical screening can choose between sampling at the clinician's office (Cervex Brush) or self-sampling at home (Evalyn Brush). HrHPV testing is performed using Roche Cobas 4800. We collected screening test results between January 2017 and March 2018 and histological follow-up until August 2019. The main outcome measures were mean cycle threshold (Ct) value, cervical intraepithelial neoplasia (CIN) grade 3 or cancer (CIN3+) and CIN grade 2 or worse (CIN2+).
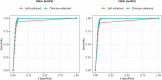
Findings: 30,808 women had a self-collected and 456,207 had a clinician-collected sample. In hrHPV-positive women with adequate cytology, Ct values were higher for self-collection than clinician-collection with a mean Ct difference of 1·25 (95% CI 0·98-1·52) in women without CIN2+, 2·73 (1·75-3·72) in CIN2 and 3·59 (3·03-4·15) in CIN3+. The relative sensitivity for detecting CIN3+ was 0·94 (0·90-0·97) for self-collection versus clinician-collection and the relative specificity was 1·02 (1·02-1·02).
Interpretation: The clinical accuracy of hrHPV testing on a self-collected sample for detection of CIN3+ is high and supports its use as a primary screening test for all invited women. Because of the slightly lower sensitivity of hrHPV testing on a self-collected compared to a clinician-collected sample, an evaluation of the workflow procedure to optimise clinical performance seems warranted.
Funding: National Institute for Public Health and the Environment (the Netherlands) and the European Commission.
Keywords: CIN3; Cervical intraepithelial neoplasia; Cervical screening; Clinical accuracy; Human papillomavirus (HPV); Routine screening; Self-sampling.
© 2021 The Authors.
Conflict of interest statement
FI and JB report grants from the European Commission (RISCC, grant number 847845) during the conduct of the study. CA, IdK, and JB report grants from the Dutch National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu, RIVM) during the conduct of the study. DAMH is minority shareholder of Self-screen B.V., a spin-off company of VUmc, which develops, manufactures and licences hrHPV and methylation marker assays for cervical cancer screening and holds patents on these tests. DAMH has been on the speakers’ bureau of Qiagen and serves occasionally on the scientific advisory boards of Pfizer and Bristol-Myers Squibb. All other authors declare no competing interests.
Figures


References
-
- Nelson EJ, Maynard BR, Loux T, Fatla J, Gordon R, Arnold LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. 2017;93(1):56–61. - PubMed
-
- Polman NJ, de Haan Y, Veldhuijzen NJ, et al. Experience with HPV self-sampling and clinician-based sampling in women attending routine cervical screening in the Netherlands. Prev Med. 2019;125:5–11. - PubMed
-
- RIVM. Monitor Bevolkingsonderzoek Baarmoederhalskanker 2018. [Published 20/12/2019. Accessed 19/03/2021]. Available from: https://www.rivm.nl/documenten/monitor-bevolkingsonderzoek-baarmoederhal....
LinkOut - more resources
Full Text Sources

