LRRK2 regulates actin assembly for spindle migration and mitochondrial function in mouse oocyte meiosis
- PMID: 34918122
- PMCID: PMC8962687
- DOI: 10.1093/jmcb/mjab079
LRRK2 regulates actin assembly for spindle migration and mitochondrial function in mouse oocyte meiosis
Erratum in
-
Erratum.J Mol Cell Biol. 2022 May 25;14(2):mjac030. doi: 10.1093/jmcb/mjac030. J Mol Cell Biol. 2022. PMID: 35639540 Free PMC article. No abstract available.
Abstract
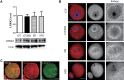
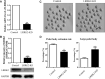
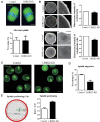
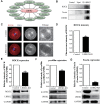
Leucine-rich-repeat kinase 2 (LRRK2) belongs to the Roco GTPase family and is a large multidomain protein harboring both GTPase and kinase activities. LRRK2 plays indispensable roles in many processes, such as autophagy and vesicle trafficking in mitosis. In this study, we showed the critical roles of LRRK2 in mammalian oocyte meiosis. LRRK2 is mainly accumulated at the meiotic spindle periphery during oocyte maturation. Depleting LRRK2 led to the polar body extrusion defects and also induced large polar bodies in mouse oocytes. Mass spectrometry analysis and co-immunoprecipitation results showed that LRRK2 was associated with several actin-regulating factors, such as Fascin and Rho-kinase (ROCK), and depletion of LRRK2 affected the expression of ROCK, phosphorylated cofilin, and Fascin. Further analysis showed that LRRK2 depletion did not affect spindle organization but caused the failure of spindle migration, which was largely due to the decrease of cytoplasmic actin filaments. Moreover, LRRK2 showed a similar localization pattern to mitochondria, and LRRK2 was associated with several mitochondria-related proteins. Indeed, mitochondrial distribution and function were both disrupted in LRRK2-depleted oocytes. In summary, our results indicated the critical roles of LRRK2 in actin assembly for spindle migration and mitochondrial function in mouse oocyte meiosis.
Keywords: LRRK2; actin; meiosis; oocyte; spindle.
© The Author(s) (2021). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures

References
-
- Almonacid M., Terret M.E., Verlhac M.H. (2014). Actin-based spindle positioning: new insights from female gametes. J. Cell Sci. 127, 477–483. - PubMed
-
- Bavister B.D., Squirrell J.M. (2000). Mitochondrial distribution and function in oocytes and early embryos. Hum. Reprod. 15 (Suppl 2), 189–198. - PubMed

