Enteropathogen Changes After Rotavirus Vaccine Scale-up
- PMID: 34918158
- PMCID: PMC9647525
- DOI: 10.1542/peds.2020-049884
Enteropathogen Changes After Rotavirus Vaccine Scale-up
Abstract
Objectives: To inform next steps in pediatric diarrhea burden reduction by understanding the shifting enteropathogen landscape after rotavirus vaccine implementation.
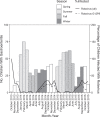
Methods: We conducted a case-control study of 1788 medically attended children younger than 5 years, with and without gastroenteritis, after universal rotavirus vaccine implementation in Peru. We tested case and control stools for 5 viruses, 19 bacteria, and parasites; calculated coinfection-adjusted attributable fractions (AFs) to determine pathogen-specific burdens; and evaluated pathogen-specific gastroenteritis severity using Clark and Vesikari scales.
Results: Six pathogens were independently positively associated with gastroenteritis: norovirus genogroup II (GII) (AF 29.1, 95% confidence interval [CI]: 28.0-32.3), rotavirus (AF 8.9, 95% CI: 6.8-9.7), sapovirus (AF 6.3, 95% CI: 4.3-7.4), astrovirus (AF 2.8, 95% CI: 0.0-4.0); enterotoxigenic Escherichia coli heat stable and/or heat labile and heat stable (AF 2.4, 95% CI: 0.6-3.1), and Shigella spp. (AF 2.0, 95% CI: 0.4-2.2). Among typeable rotavirus cases, we most frequently identified partially heterotypic strain G12P[8] (54 of 81, 67%). Mean severity was significantly higher for norovirus GII-positive cases relative to norovirus GII-negative cases (Vesikari [12.7 vs 11.8; P < .001] and Clark [11.7 vs 11.4; P = .016]), and cases in the 6- to 12-month age range relative to cases in other age groups (Vesikari [12.7 vs 12.0; P = .0002] and Clark [12.0 vs 11.4; P = .0016]).
Conclusions: Norovirus is well recognized as the leading cause of pediatric gastroenteritis in settings with universal rotavirus vaccination. However, sapovirus is often overlooked. Both norovirus and sapovirus contribute significantly to the severe pediatric disease burden in this setting. Decision-makers should consider multivalent vaccine acquisition strategies to target multiple caliciviruses in similar countries after successful rotavirus vaccine implementation.
Some authors are military service members or employees of the US Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United states Government.” Title 17 USC §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Conflict of interest statement
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Figures


References
-
- Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222 - PubMed
-
- Platts-Mills JA, Liu J, Rogawski ET, et al. ; MAL-ED Network Investigators . Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018;6(12):e1309–e1318 - PMC - PubMed
-
- Rouhani S, Peñataro Yori P, Paredes Olortegui M, et al. ; Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) Network Investigators . Norovirus infection and acquired immunity in 8 countries: results from the MAL-ED study. Clin Infect Dis. 2016;62(10):1210–1217 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

