Early outcomes of transcatheter mitral valve replacement with the Tendyne system in severe mitral annular calcification
- PMID: 34918624
- PMCID: PMC9896404
- DOI: 10.4244/EIJ-D-21-00745
Early outcomes of transcatheter mitral valve replacement with the Tendyne system in severe mitral annular calcification
Abstract
Background: Treatment of mitral regurgitation (MR) associated with severe mitral annular calcification (MAC) is challenging due to the high risk of fatal atrioventricular groove disruption and significant paravalvular leak.
Aims: The aim of this study was to evaluate the outcomes of transcatheter mitral valve replacement (TMVR) with the Tendyne valve (Abbott Structural) in patients with MR and MAC.
Methods: Twenty patients (mean age 78 years; 11 women) who were treated with the Tendyne valve, either compassionate use (CU; closed) or as part of The Feasibility Study of Tendyne in MAC (NCT03539458), had reported outcomes in a median follow-up duration of 368 days.
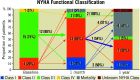
Results: In all patients, a valve was implanted with no procedural mortality and successful hospital discharge. Two embolic events occurred, including one with mesenteric ischaemia and one non-disabling stroke. At 30 days and one year, all-cause mortality occurred in one (5%) and eight patients (40%), respectively. At one year, six patients had been hospitalised for heart failure (30%). There was no prosthetic dysfunction, and MR remained absent in all patients at one year. Clinical improvement, measured by New York Heart Association Functional Class, occurred in 11 of 12 patients who were alive at one year. Among seven survivors with Kansas City Cardiomyopathy Questionnaire (KCCQ) data, mean increase in KCCQ score was 29.9±26.3 at one year with improvement of ≥10 points in five (71.4%) patients.
Conclusions: In patients with MR and severe MAC, TMVR with the Tendyne valve was associated with encouraging acute outcomes, midterm durability, and clinical improvement. Dedicated TMVR therapy may have a future role in these anatomically challenging, high-risk patients.
Conflict of interest statement
M. Gössl reports consulting for Abbott Vascular, and is Chair of the Subject Eligibility Committee for the SUMMIT trial. V. Thourani reports consulting and research for Abbott Vascular. V. Babaliaros reports consulting for Abbott (less than $5,000), and Edwards Lifesciences. L. Conradi reports being a member of the advisory board for Abbott Vascular, and being a consultant for Boston Scientific, Edwards Lifesciences, and Medtronic. B. Chehab reports being a member of the advisory board and a consultant for Abbott Structural, Edwards Lifesciences, and Medtronic. N. Dumonteil reports consulting and proctoring fees for Abbott Vascular, Ancora Heart, Boston Scientific, Edwards Lifesciences and Medtronic. V. Badhwar reports institutional research support from and being a consultant (non-remunerative) for Abbott. D. Rizik reports being on the Medical Advisory Board of Abbott Vascular, and being on the Executive Physician Council of Boston Scientific. B. Sun reports being a consultant and clinical proctor for Abbott Vascular and being a selection committee member for the Tendyne Device (Summit Clinical Trial). R. Bae reports consulting for Abbott Vascular - Speakers Bureau. R. Guyton reports being a consultant for Edwards Lifesciences (no consultation or compensation in last 12 months). M. Chuang is an employee of the Beth Israel Deaconess Medical Center Cardiovascular Imaging Core Laboratory which received funds from the study sponsor to perform image analyses. He also reports Echo Core Laboratory services for Abbott Vascular. P. Blanke reports being a consultant for Edwards Lifesciences and W.L. Gore, CT Core Laboratory services for Abbott Vascular, institutional core laboratory services for Abbott Laboratories, related to this manuscript, with no direct personal compensation, and institutional core laboratory services not related to this manuscript for Medtronic, Edwards Lifesciences, Boston Scientific, and Neovasc without direct personal compensation. P. Sorajja reports consulting or advisory board participation for Abbott Structural, Anteris, Boston Scientific, Medtronic, NeoVasc, TriFlo, VDyne, and W.L. Gore.
Figures





References
-
- Gillinov AM, Desai MY, Mick S. Mitral Annular Calcification: The Search For Safer Options. J Am Coll Cardiol. 2018;72:1449–51. - PubMed
-
- Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral Annulus Calcification. J Am Coll Cardiol. 2015;66:1934–41. - PubMed
-
- Bedeir K, Kaneko T, Aranki S. Current and evolving strategies in the management of severe mitral annular calcification. J Thorac Cardiovasc Surg. 2019;157:555–66. - PubMed
-
- Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, Pershad A, Fang HK, O'Hair D, Jones N, Mahadevan VS, Dumonteil N, Rodés-Cabau J, Piazza N, Ferrari E, Ciaburri D, Nejjari M, DeLago A, Sorajja P, Zahr F, Rajagopal V, Whisenant B, Shah PB, Sinning JM, Witkowski A, Eltchaninoff H, Dvir D, Martin B, Attizzani GF, Gaia D, Nunes NSV, Fassa AA, Kerendi F, Pavlides G, Iyer V, Kaddissi G, Witzke C, Wudel J, Mishkel G, Raybuck B, Wang C, Waksman R, Palacios I, Cribier A, Webb J, Bapat V, Reisman M, Makkar R, Leon M, Rihal C, Vahanian A, O'Neill W, Feldman T. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. J Am Coll Cardiol. 2018;71:1841–53. - PubMed
-
- Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, Kodali S, George I, Dvir D, Whisenant B, Russo MJ, Pershad A, Fang K, Coylewright M, Shah P, Babaliaros V, Khan JM, Tommaso C, Saucedo J, Kar S, Makkar R, Mack M, Holmes D, Leon M, Bapat V, Thourani VH, Rihal C, O'Neill W, Feldman T. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

