Pick your prior: scepticism about sceptical prior beliefs
- PMID: 34919155
- PMCID: PMC8678426
- DOI: 10.1007/s00134-021-06602-z
Pick your prior: scepticism about sceptical prior beliefs
Conflict of interest statement
Both authors declare to have no conflict of interest.
Figures
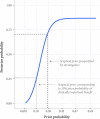
Comment in
-
Choice of priors: how much scepticism is appropriate?Intensive Care Med. 2022 Mar;48(3):372-373. doi: 10.1007/s00134-021-06613-w. Epub 2022 Jan 13. Intensive Care Med. 2022. PMID: 35024884 No abstract available.
Comment on
-
Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial.Intensive Care Med. 2022 Jan;48(1):45-55. doi: 10.1007/s00134-021-06573-1. Epub 2021 Nov 10. Intensive Care Med. 2022. PMID: 34757439 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources

