Configurationally Chiral SuFEx-Based Polymers
- PMID: 34919320
- PMCID: PMC9303861
- DOI: 10.1002/anie.202116158
Configurationally Chiral SuFEx-Based Polymers
Abstract
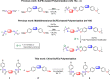
Novel methods to make synthetic chiral polymers are highly desirable given their potential in a rapidly increasing number of bio-inspired applications. The enantiospecific sulfur-fluorine exchange (SuFEx) reaction of chiral di-sulfonimidoyl fluorides (di-SFs) with diphenols, was used to produce high-molecular-weight chiral polymers with configurational backbone chirality. The resulting new class of polymers, polysulfonimidates, can be efficiently produced via this step-growth mechanism for a wide range of di-SFs and diphenols, yielding MnPS up to 283 kDa with a typical dispersity Đ around 1.6. The optical activity of the resulting chiral polymers is largely due to the intrinsic asymmetry of the S atoms (configurational chirality). Finally, the enantiospecificity (ee>98 %) of the polymerization reaction was demonstrated by the degradation of a disulfide-containing polysulfonimidate. This novel route towards configurational main-chain chirality opens up new approaches towards tailor-made chiral polymers with precisely defined properties.
Keywords: Chiral Polymer; Click Chemistry; Enantiospecific; Polysulfonimidates; SuFEx Reaction.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zhang Y., Deng J., Polym. Chem. 2020, 11, 5407–5423.
-
- Itsuno S., Parvez M. M., Haraguchi N., Polym. Chem. 2011, 2, 1942–1949.
-
- Shen J., Okamoto Y., Chem. Rev. 2016, 116, 1094–1138. - PubMed
-
- Li M., Qing G., Zhang M., Sun T., Sci. China Chem. 2014, 57, 540–551.
-
- None
Publication types
LinkOut - more resources
Full Text Sources

