Bis-Rhodamines Bridged with a Diazoketone Linker: Synthesis, Structure, and Photolysis
- PMID: 34919387
- PMCID: PMC8749961
- DOI: 10.1021/acs.joc.1c01721
Bis-Rhodamines Bridged with a Diazoketone Linker: Synthesis, Structure, and Photolysis
Abstract
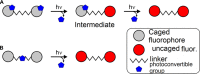
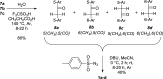
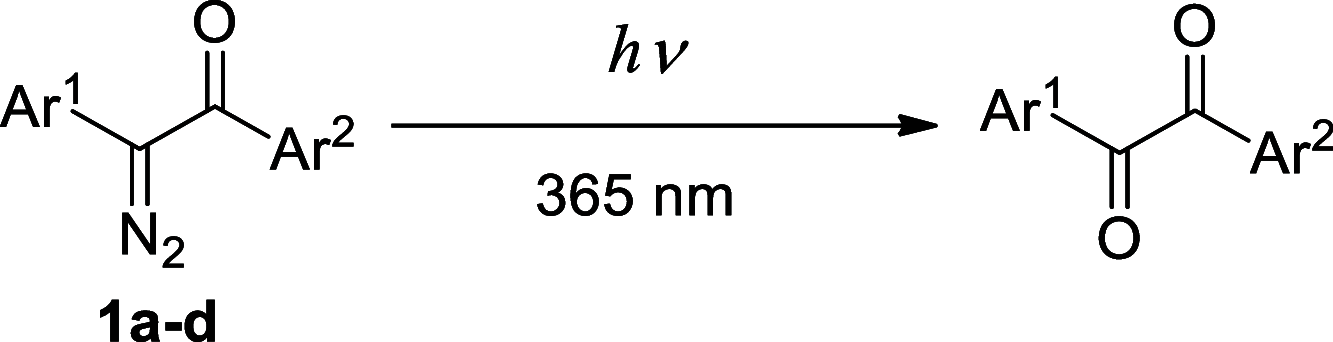
Two fluorophores bound with a short photoreactive bridge are fascinating structures and remained unexplored. To investigate the synthesis and photolysis of such dyes, we linked two rhodamine dyes via a diazoketone bridge (-COCN2-) attached to position 5' or 6' of the pendant phenyl rings. For that, the mixture of 5'- or 6'-bromo derivatives of the parent dye was prepared, transformed into 1,2-diarylacetylenes, hydrated to 1,2-diarylethanones, and converted to diazoketones Ar1COCN2Ar2. The high performance liquid chromatography (HPLC) separation gave four individual regioisomers of Ar1COCN2Ar2. Photolysis of the model compound─C6H5COCN2C6H5─in aqueous acetonitrile at pH 7.3 and under irradiation with 365 nm light provided diphenylacetic acid amide (Wolff rearrangement). However, under the same conditions, Ar1COCN2Ar2 gave mainly α-diketones Ar1COCOAr2. The migration ability of the very bulky dye residues was low, and the Wolff rearrangement did not occur. We observed only moderate fluorescence increase, which may be explained by the insufficient quenching ability of diazoketone bridge (-COCN2-) and its transformation into another (weaker) quencher, 1,2-diarylethane-1,2-dione.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


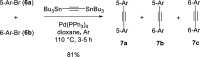



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

