Inactive-to-Active Transition of Human Thymidine Kinase 1 Revealed by Molecular Dynamics Simulations
- PMID: 34919400
- PMCID: PMC8757434
- DOI: 10.1021/acs.jcim.1c01157
Inactive-to-Active Transition of Human Thymidine Kinase 1 Revealed by Molecular Dynamics Simulations
Abstract
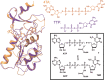
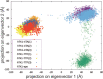
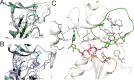
Despite its importance in the nucleoside (and nucleoside prodrug) metabolism, the structure of the active conformation of human thymidine kinase 1 (hTK1) remains elusive. We perform microsecond molecular dynamics simulations of the inactive enzyme form bound to a bisubstrate inhibitor that was shown experimentally to activate another TK1-like kinase, Thermotoga maritima TK (TmTK). Our results are in excellent agreement with the experimental findings for the TmTK closed-to-open state transition. We show that the inhibitor induces an increase of the enzyme radius of gyration due to the expansion on one of the dimer interfaces; the structural changes observed, including the active site pocket volume increase and the decrease in the monomer-monomer buried surface area and of the number of hydrogen bonds (as compared to the inactive enzyme control simulation), indicate that the catalytically competent (open) conformation of hTK1 can be assumed in the presence of an activating ligand.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Binding of ATP to TK1-like enzymes is associated with a conformational change in the quaternary structure.J Mol Biol. 2007 May 25;369(1):129-41. doi: 10.1016/j.jmb.2007.02.104. Epub 2007 Mar 15. J Mol Biol. 2007. PMID: 17407781 Free PMC article.
-
Quaternary structure change as a mechanism for the regulation of thymidine kinase 1-like enzymes.Structure. 2007 Dec;15(12):1555-66. doi: 10.1016/j.str.2007.09.025. Structure. 2007. PMID: 18073106 Free PMC article.
-
Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells.J Biol Chem. 2004 Jul 30;279(31):32832-8. doi: 10.1074/jbc.M313343200. Epub 2004 May 25. J Biol Chem. 2004. PMID: 15163659
-
N3-substituted thymidine bioconjugates for cancer therapy and imaging.Future Med Chem. 2013 Apr;5(6):677-92. doi: 10.4155/fmc.13.31. Future Med Chem. 2013. PMID: 23617430 Free PMC article. Review.
-
Integrated homology modelling and X-ray study of herpes simplex virus I thymidine kinase: a case study.J Recept Signal Transduct Res. 1997 Jan-May;17(1-3):475-94. doi: 10.3109/10799899709036622. J Recept Signal Transduct Res. 1997. PMID: 9029509 Review.
Cited by
-
Allostery and Evolution: A Molecular Journey Through the Structural and Dynamical Landscape of an Enzyme Super Family.Mol Biol Evol. 2025 Jan 6;42(1):msae265. doi: 10.1093/molbev/msae265. Mol Biol Evol. 2025. PMID: 39834309 Free PMC article.
-
Protein Conformational Space at the Edge of Allostery: Turning a Nonallosteric Malate Dehydrogenase into an "Allosterized" Enzyme Using Evolution-Guided Punctual Mutations.Mol Biol Evol. 2022 Sep 1;39(9):msac186. doi: 10.1093/molbev/msac186. Mol Biol Evol. 2022. PMID: 36056899 Free PMC article.
References
-
- Jagiello K.; Makurat S.; Pereć S.; Rak J.; Puzyn T. Molecular features of thymidine analogues governing the activity of human thymidine kinase. Struct. Chem. 2018, 29, 1367–1374. 10.1007/s11224-018-1124-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

