A phase II study repurposing atomoxetine for neuroprotection in mild cognitive impairment
- PMID: 34919634
- PMCID: PMC9630662
- DOI: 10.1093/brain/awab452
A phase II study repurposing atomoxetine for neuroprotection in mild cognitive impairment
Abstract
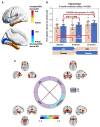
The locus coeruleus is the initial site of Alzheimer's disease neuropathology, with hyperphosphorylated Tau appearing in early adulthood followed by neurodegeneration in dementia. Locus coeruleus dysfunction contributes to Alzheimer's pathobiology in experimental models, which can be rescued by increasing norepinephrine transmission. To test norepinephrine augmentation as a potential disease-modifying therapy, we performed a biomarker-driven phase II trial of atomoxetine, a clinically-approved norepinephrine transporter inhibitor, in subjects with mild cognitive impairment due to Alzheimer's disease. The design was a single-centre, 12-month double-blind crossover trial. Thirty-nine participants with mild cognitive impairment and biomarker evidence of Alzheimer's disease were randomized to atomoxetine or placebo treatment. Assessments were collected at baseline, 6- (crossover) and 12-months (completer). Target engagement was assessed by CSF and plasma measures of norepinephrine and metabolites. Prespecified primary outcomes were CSF levels of IL1α and TECK. Secondary/exploratory outcomes included clinical measures, CSF analyses of amyloid-β42, Tau, and pTau181, mass spectrometry proteomics and immune-based targeted inflammation-related cytokines, as well as brain imaging with MRI and fluorodeoxyglucose-PET. Baseline demographic and clinical measures were similar across trial arms. Dropout rates were 5.1% for atomoxetine and 2.7% for placebo, with no significant differences in adverse events. Atomoxetine robustly increased plasma and CSF norepinephrine levels. IL-1α and TECK were not measurable in most samples. There were no significant treatment effects on cognition and clinical outcomes, as expected given the short trial duration. Atomoxetine was associated with a significant reduction in CSF Tau and pTau181 compared to placebo, but not associated with change in amyloid-β42. Atomoxetine treatment also significantly altered CSF abundances of protein panels linked to brain pathophysiologies, including synaptic, metabolism and glial immunity, as well as inflammation-related CDCP1, CD244, TWEAK and osteoprotegerin proteins. Treatment was also associated with significantly increased brain-derived neurotrophic factor and reduced triglycerides in plasma. Resting state functional MRI showed significantly increased inter-network connectivity due to atomoxetine between the insula and the hippocampus. Fluorodeoxyglucose-PET showed atomoxetine-associated increased uptake in hippocampus, parahippocampal gyrus, middle temporal pole, inferior temporal gyrus and fusiform gyrus, with carry-over effects 6 months after treatment. In summary, atomoxetine treatment was safe, well tolerated and achieved target engagement in prodromal Alzheimer's disease. Atomoxetine significantly reduced CSF Tau and pTau, normalized CSF protein biomarker panels linked to synaptic function, brain metabolism and glial immunity, and increased brain activity and metabolism in key temporal lobe circuits. Further study of atomoxetine is warranted for repurposing the drug to slow Alzheimer's disease progression.
Keywords: Alzheimer’s disease; atomoxetine; locus coeruleus; mild cognitive impairment; norepinephrine.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Marcyniuk B, Mann DM, Yates PO. The topography of cell loss from locus caeruleus in Alzheimer’s disease. J Neurol Sci. 1986;76(2–3):335–345. - PubMed
-
- Palmer AM, Francis PT, Bowen DM, et al. Catecholaminergic neurones assessed ante-mortem in Alzheimer’s disease. Brain Res. 1987;414(2):365–375. - PubMed
-
- Palmer AM, Wilcock GK, Esiri MM, Francis PT, Bowen DM. Monoaminergic innervation of the frontal and temporal lobes in Alzheimer’s disease. Brain Res. 1987;401(2):231–238. - PubMed

