COVID-19 and Immune-Mediated RBC Destruction
- PMID: 34919640
- PMCID: PMC8755306
- DOI: 10.1093/ajcp/aqab210
COVID-19 and Immune-Mediated RBC Destruction
Abstract
Objectives: To summarize the epidemiologic, clinical, and laboratory characteristics of autoimmune hemolytic anemia (AIHA) secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or vaccination.
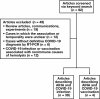
Methods: We conducted a systematic review using standardized keyword search to identify all reports of SARS-CoV-2 infection or vaccination and AIHA across PubMed, Web of Science, Scopus, and Google Scholar through September 24, 2021.
Results: Fifty patients (mean [SD] age, 50.8 [21.6] years) diagnosed with coronavirus disease 2019 (COVID-19) and AIHA were identified. AIHA subtypes and number of patients were as follows: cold AIHA (n = 18), warm AIHA (n = 14), mixed-type AIHA (n = 3), direct antiglobulin test (DAT)-negative AIHA (n = 1), DAT-negative Evans syndrome (n = 1), Evans syndrome (n = 3), and subtype not reported (n = 10). Mean (SD) hemoglobin at AIHA diagnosis was 6.5 [2.8] g/dL (95% confidence interval, 5.7-7.3 g/dL). Median time from COVID-19 symptom onset to AIHA diagnosis was 7 days. In total, 19% (8/42) of patients with COVID-19-associated AIHA with reported outcomes were deceased. Four patients (mean [SD] age, 73.5 [16.9] years) developed AIHA following SARS-CoV-2 vaccination: Pfizer-BioNTech BNT162b2 vaccine (n = 2); Moderna mRNA-1273 vaccine (n = 1); undisclosed mRNA vaccine (n = 1). AIHA occurred after 1 dose in 3 patients (median, 5 days).
Conclusions: SARS-CoV-2 infection and vaccination are associated with multiple AIHA subtypes, beginning approximately 7 days after infectious symptoms and 5 days after vaccination.
Keywords: Autoimmune hemolytic anemia; COVID-19; Cold agglutinin disease; Hemolysis; SARS-CoV-2; SARS-CoV-2 vaccine.
© American Society for Clinical Pathology, 2021. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous