Cortical and subcortical pathological burden and neuronal loss in an autopsy series of FTLD-TDP-type C
- PMID: 34919645
- PMCID: PMC9050539
- DOI: 10.1093/brain/awab368
Cortical and subcortical pathological burden and neuronal loss in an autopsy series of FTLD-TDP-type C
Abstract
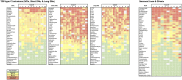
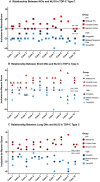
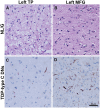
The TDP-43 type C pathological form of frontotemporal lobar degeneration is characterized by the presence of immunoreactive TDP-43 short and long dystrophic neurites, neuronal cytoplasmic inclusions, neuronal loss and gliosis and the absence of neuronal intranuclear inclusions. Frontotemporal lobar degeneration-TDP-type C cases are commonly associated with the semantic variant of primary progressive aphasia or behavioural variant frontotemporal dementia. Here, we provide detailed characterization of regional distributions of pathological TDP-43 and neuronal loss and gliosis in cortical and subcortical regions in 10 TDP-type C cases and investigate the relationship between inclusions and neuronal loss and gliosis. Specimens were obtained from the first 10 TDP-type C cases accessioned from the Northwestern Alzheimer's Disease Research Center (semantic variant of primary progressive aphasia, n = 7; behavioural variant frontotemporal dementia, n = 3). A total of 42 cortical (majority bilateral) and subcortical regions were immunostained with a phosphorylated TDP-43 antibody and/or stained with haematoxylin-eosin. Regions were evaluated for atrophy, and for long dystrophic neurites, short dystrophic neurites, neuronal cytoplasmic inclusions, and neuronal loss and gliosis using a semiquantitative 5-point scale. We calculated a 'neuron-to-inclusion' score (TDP-type C mean score - neuronal loss and gliosis mean score) for each region per case to assess the relationship between TDP-type C inclusions and neuronal loss and gliosis. Primary progressive aphasia cases demonstrated leftward asymmetry of cortical atrophy consistent with the aphasic phenotype. We also observed abundant inclusions and neurodegeneration in both cortical and subcortical regions, with certain subcortical regions emerging as particularly vulnerable to dystrophic neurites (e.g. amygdala, caudate and putamen). Interestingly, linear mixed models showed that regions with lowest TDP-type C pathology had high neuronal dropout, and conversely, regions with abundant pathology displayed relatively preserved neuronal densities (P < 0.05). This inverse relationship between the extent of TDP-positive inclusions and neuronal loss may reflect a process whereby inclusions disappear as their associated neurons are lost. Together, these findings offer insight into the putative substrates of neurodegeneration in unique dementia syndromes.
Keywords: TDP-43; behavioural variant frontotemporal dementia; frontotemporal lobar degeneration; primary progressive aphasia.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Cairns NJ, Bigio EH, Mackenzie IR, et al. ; Consortium for Frontotemporal Lobar Degeneration. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5–22. - PMC - PubMed
-
- Mesulam MM. Primary progressive aphasia—a language-based dementia. N Engl J Med. 2003;349(16):1535–1542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

