Antibody response to SARS-CoV-2 for more than one year - kinetics and persistence of detection are predominantly determined by avidity progression and test design
- PMID: 34920374
- PMCID: PMC8642248
- DOI: 10.1016/j.jcv.2021.105052
Antibody response to SARS-CoV-2 for more than one year - kinetics and persistence of detection are predominantly determined by avidity progression and test design
Abstract
Background: Antibody detection of SARS-CoV-2 requires an understanding of its variation, course, and duration.
Methods: Antibody response to SARS-CoV-2 was evaluated over 5-430 days on 828 samples across COVID-19 severity levels, for total antibody (TAb), IgG, IgA, IgM, neutralizing antibody (NAb), antibody avidity, and for receptor-binding-domain (RBD), spike (S), or nucleoprotein (N). Specificity was determined on 676 pre-pandemic samples.
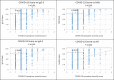
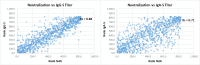
Results: Sensitivity at 30-60 days post symptom onset (pso) for TAb-S/RBD, TAb-N, IgG-S, IgG-N, IgA-S, IgM-RBD, and NAb was 96.6%, 99.5%, 89.7%, 94.3%, 80.9%, 76.9% and 92.8%, respectively. Follow-up 430 days pso revealed: TAb-S/RBD increased slightly (100.0%); TAb-N decreased slightly (97.1%); IgG-S and IgA-S decreased moderately (81.4%, 65.7%); NAb remained positive (94.3%), slightly decreasing in activity after 300 days; there was correlation with IgG-S (Rs = 0.88) and IgA-S (Rs = 0.71); IgG-N decreased significantly from day 120 (15.7%); IgM-RBD dropped after 30-60 days (22.9%). High antibody avidity developed against S/RBD steadily with time in 94.3% of patients after 430 days. This correlated with persistent antibody detection depending on antibody-binding efficiency of the test design. Severe COVID-19 correlated with earlier and higher antibody response, mild COVID-19 was heterogeneous with a wide range of antibody reactivities. Specificity of the tests was ≥99%, except for IgA (96%).
Conclusion: Sensitivity of anti-SARS-CoV-2 assays was determined by test design, target antigen, antibody avidity, and COVID-19 severity. Sustained antibody detection was mainly determined by avidity progression for RBD and S. Testing by TAb and for S/RBD provided the highest sensitivity and longest detection duration of 14 months so far.
Keywords: Antibody avidity; COVID-19; Persistence SARS-CoV-2 antibodies; Sensitivity; Specificity; neutralization.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interests.
Figures




References
-
- Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

