SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury
- PMID: 34921131
- PMCID: PMC8677926
- DOI: 10.1038/s41392-021-00849-0
SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury
Erratum in
-
Correction: SARS-CoV-2-triggered mast cell rapid degranulation induces alveolar epithelial inflammation and lung injury.Signal Transduct Target Ther. 2025 Oct 31;10(1):359. doi: 10.1038/s41392-025-02465-8. Signal Transduct Target Ther. 2025. PMID: 41173839 No abstract available.
Abstract
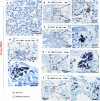
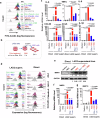
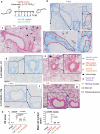
SARS-CoV-2 infection-induced hyper-inflammation links to the acute lung injury and COVID-19 severity. Identifying the primary mediators that initiate the uncontrolled hypercytokinemia is essential for treatments. Mast cells (MCs) are strategically located at the mucosa and beneficially or detrimentally regulate immune inflammations. In this study, we showed that SARS-CoV-2-triggered MC degranulation initiated alveolar epithelial inflammation and lung injury. SARS-CoV-2 challenge induced MC degranulation in ACE-2 humanized mice and rhesus macaques, and a rapid MC degranulation could be recapitulated with Spike-RBD binding to ACE2 in cells; MC degranulation altered various signaling pathways in alveolar epithelial cells, particularly, the induction of pro-inflammatory factors and consequential disruption of tight junctions. Importantly, the administration of clinical MC stabilizers for blocking degranulation dampened SARS-CoV-2-induced production of pro-inflammatory factors and prevented lung injury. These findings uncover a novel mechanism for SARS-CoV-2 initiating lung inflammation, and suggest an off-label use of MC stabilizer as immunomodulators for COVID-19 treatments.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- O’Driscoll, M. et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature590, 140–145 (2021). - PubMed
-
- Rubin, D., Chan-Tack, K., Farley, J. & Sherwat, A. FDA approval of remdesivir - a step in the right direction. N. Engl. J. Med383, 2598–2600 (2020). - PubMed
-
- Mahase, E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ375, n2697 (2021). - PubMed
-
- Mahase, E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ375, n2422 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

