Comparative Effectiveness and Cost-Effectiveness of Natalizumab and Fingolimod in Patients with Inadequate Response to Disease-Modifying Therapies in Relapsing-Remitting Multiple Sclerosis in the United Kingdom
- PMID: 34921350
- PMCID: PMC8866337
- DOI: 10.1007/s40273-021-01106-6
Comparative Effectiveness and Cost-Effectiveness of Natalizumab and Fingolimod in Patients with Inadequate Response to Disease-Modifying Therapies in Relapsing-Remitting Multiple Sclerosis in the United Kingdom
Abstract
Background: Patients with highly active relapsing-remitting multiple sclerosis inadequately responding to first-line therapies (interferon-based therapies, glatiramer acetate, dimethyl fumarate, and teriflunomide, known collectively as "BRACETD") often switch to natalizumab or fingolimod.
Objective: The aim was to estimate the comparative effectiveness of switching to natalizumab or fingolimod or within BRACETD using real-world data and to evaluate the cost-effectiveness of switching to natalizumab versus fingolimod using a United Kingdom (UK) third-party payer perspective.
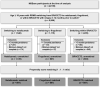
Methods: Real-world data were obtained from MSBase for patients relapsing on BRACETD in the year before switching to natalizumab or fingolimod or within BRACETD. Three-way-multinomial-propensity-score-matched cohorts were identified, and comparisons between treatment groups were conducted for annualised relapse rate (ARR) and 6-month-confirmed disability worsening (CDW6M) and improvement (CDI6M). Results were applied in a cost-effectiveness model over a lifetime horizon using a published Markov structure with health states based on the Expanded Disability Status Scale. Other model parameters were obtained from the UK MS Survey 2015, published literature, and publicly available UK sources.
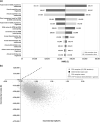
Results: The MSBase analysis found a significant reduction in ARR (rate ratio [RR] = 0.64; 95% confidence interval [CI] 0.57-0.72; p < 0.001) and an increase in CDI6M (hazard ratio [HR] = 1.67; 95% CI 1.30-2.15; p < 0.001) for switching to natalizumab compared with BRACETD. For switching to fingolimod, the reduction in ARR (RR = 0.91; 95% CI 0.81-1.03; p = 0.133) and increase in CDI6M (HR = 1.30; 95% CI 0.99-1.72; p = 0.058) compared with BRACETD were not significant. Switching to natalizumab was associated with a significant reduction in ARR (RR = 0.70; 95% CI 0.62-0.79; p < 0.001) and an increase in CDI6M (HR = 1.28; 95% CI 1.01-1.62; p = 0.040) compared to switching to fingolimod. No evidence of difference in CDW6M was found between treatment groups. Natalizumab dominated (higher quality-adjusted life-years [QALYs] and lower costs) fingolimod in the base-case cost-effectiveness analysis (0.453 higher QALYs and £20,843 lower costs per patient). Results were consistent across sensitivity analyses.
Conclusions: This novel real-world analysis suggests a clinical benefit for therapy escalation to natalizumab versus fingolimod based on comparative effectiveness results, translating to higher QALYs and lower costs for UK patients inadequately responding to BRACETD.
© 2021. The Author(s).
Conflict of interest statement
Timothy Spelman is a statistical contractor for the MSBase Foundation and has received compensation for serving on advisory boards for Biogen and speaker honoraria from Novartis and Roche. William L. Herring, Yuanhui Zhang, and Isobel Pearson are employees of RTI Health Solutions, an independent non-profit research organisation, which received funding pursuant to a contract with Biogen. Michael Tempest, Ulrich Freudensprung, Carlos Acosta, and Robert Hyde are employees of Biogen and hold shares or stocks from Biogen as part of their remuneration. Thibaut Dort was an employee and shareholder of Biogen at the time of his passing. Eva Havrdova has received honoraria and research support from Biogen, Merck Serono, Novartis, Roche, and Teva; has served in advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novartis, and Sanofi Genzyme; and has been supported by the Czech Ministry of Education research project PROGRES Q27/LF1. Dana Horakova has received speaker honoraria and consulting fees from Biogen, Merck, Novartis, Roche, Sanofi Genzyme, and Teva and has received support for research activities from Biogen and the Czech Ministry of Education (project Progres Q27/LF1). Maria Trojano has served on scientific advisory boards for Biogen, Novartis, Roche, and Merck; has received speaker honoraria from Biogen, Sanofi, Merck, Roche, Teva, and Novartis; and has received research grants for her Department from Biogen, Merck, Roche, and Novartis. Giovanna De Luca has served on scientific advisory boards for Merck, Biogen, Novartis, Roche, and Sanofi Genzyme and has received funding for travel and speaker honoraria from Biogen, Merck, Novartis, Sanofi Genzyme, and Roche. Alessandra Lugaresi has received personal compensation for consulting, serving on a scientific advisory board, speaking or other activities from Biogen, Merck Serono, Mylan, Novartis, Roche, Sanofi/Genzyme, and Teva. Her institutions have received research grants from Novartis. Guillermo Izquierdo has received speaking honoraria from Almirall, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. Pierre Grammond has served in advisory boards for Novartis, EMD Serono, Roche, Biogen, Sanofi Genzyme, and Pendopharm and has received grant support from Genzyme and Roche; his institution has also received research grants from Biogen, Sanofi Genzyme, and EMD Serono. Pierre Duquette has received support for organised continuing medical education activities and travel fees to attend advisory meetings from Biogen, EMD Serono, Genzyme, and Novartis. Raed Alroughani has received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche, Sanofi, and Genzyme. Eugenio Pucci has received travel grants from Roche, Novartis, Merck, Genzyme-Sanofi, Biogen, and Teva and received equipment from
Figures


References
-
- Butzkueven H, Chapman J, Cristiano E, et al. MSBase: an international, online registry and platform for collaborative outcomes research in multiple sclerosis. Mult Scler. 2006;12(6):769–774. - PubMed
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–616. - PubMed
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. - PubMed
-
- Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518. - PubMed

