Safety and tolerability of combination therapy with ambrisentan and tadalafil for the treatment of pulmonary arterial hypertension in children: Real-world experience
- PMID: 34921523
- PMCID: PMC8854334
- DOI: 10.1002/ppul.25796
Safety and tolerability of combination therapy with ambrisentan and tadalafil for the treatment of pulmonary arterial hypertension in children: Real-world experience
Abstract
Objective: To describe the safety and tolerability of treatment with ambrisentan and tadalafil in pediatric pulmonary hypertension (PH).
Study design: This retrospective observational two-center study included subjects (≤18 years of age) with PH receiving combination therapy with ambrisentan and tadalafil. Before initiating this therapy, many patients were on other therapies for PH. At baseline, patients either received no therapy or monotherapy with a phosphodiesterase 5 inhibitor (PDE5i) or endothelin receptor antagonist (ERA) (Group A), switched from a different PDE5i and ERA (Group B), or were on prostanoid therapy with or without a PDE5i and/or ERA (Group C and D). Demographics, symptoms, and adverse effects were collected. Pre- and postvalues for exercise capacity, hemodynamics, and biomarkers were compared.
Results: There were 43 subjects (26 F, 17 M) ages 4-17.5 years (median 9.3) with World Symposium of PH group 1, 3, and 5. Significant improvements were seen in change scores at follow-up in the entire sample and Group A for 6-min walk distance: +37.0 (6.5-71.0) [p = 0.022], mean pulmonary artery pressure: -6.0 (-14.0 to -3.5) [p = .002], pulmonary vascular resistance: -1.7 (-6.2 to -1.0) [p = .003], NT-proBNP -32.9 (-148.9 to -6.7) [p = .025]. WHO functional class improved in 39.5% and was unchanged in 53.5%; PH risk scores improved in 16%; were unchanged in 56%; and declined in 14%. Three patients discontinued therapy (two headaches, one peripheral edema). Seven patients were hospitalized for worsening disease (2/7 had a Potts shunt placed, 2/7 had an atrial septostomy). There were no deaths or lung transplantation.
Conclusions: Combination therapy with ambrisentan and tadalafil was well-tolerated, with an acceptable safety profile in a select group of children. This therapy was associated with improved exercise capacity and hemodynamics in children who were treatment naïve or on monotherapy with a PH medication before the initiation of ambrisentan and tadalafil. Based on these early data, further study of combination therapy in pediatric PH is warranted.
Keywords: ambrisentan; congenital heart disease; endothelin receptor antagonist; hemodynamics; pediatric cardiology; phosphodiesterase 5 inhibitor; pulmonary hypertension; tadalafil; targeted therapy.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
No conflicts exist for the following authors: A. A. I., S. C., M. D. H., M. D., B. F.
Author D. D. I.: The University of Colorado contracts with Actelion, Bayer, Gilead, GSK, Janssen, Lilly, and United Therapeutics for D. D. I. to be a consultant and perform clinical trials.
Author U. S. K. and E. B. R.: Columbia University has received research support from Actelion, Janssen and United Therapeutics for U. S. K. and E. B. R. to consult and perform clinical trials.
Author S. C. receives salary support from the Babies Heart Fund.
Figures

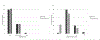

References
-
- Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. - PubMed
-
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau S, Peacock A, Vonk-Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016;37(1):67–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

