Filanesib plus bortezomib and dexamethasone in relapsed/refractory t(11;14) and 1q21 gain multiple myeloma
- PMID: 34921527
- PMCID: PMC8729045
- DOI: 10.1002/cam4.4451
Filanesib plus bortezomib and dexamethasone in relapsed/refractory t(11;14) and 1q21 gain multiple myeloma
Abstract
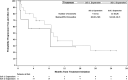
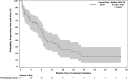
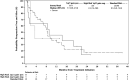
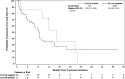
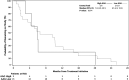
Filanesib is a first-in-class kinesin spindle protein inhibitor which demonstrated safety and encouraging activity in combination with bortezomib and dexamethasone in relapsed/refractory multiple myeloma in a preliminary analysis of dose-escalation phase results. This multicenter study included first a dose-escalation phase to determine maximum tolerated dose of two schedules of filanesib, bortezomib, and dexamethasone and a subsequent dose-expansion phase using the maximum tolerated doses. In the dose-expansion phase, 28 patients were evaluable for safety and efficacy. The most common grade ≥3 adverse events were neutropenia (21%) and anemia (18%), which were noncumulative and reversible, and hypertension (18%). The overall response rate was 43% with median duration of response not yet reached (range, 2.8-23.7+ months) with median follow-up of 6.3 months. A post hoc analysis incorporated 29 dose-escalation phase patients who received therapeutic filanesib doses, with an overall response rate of 39% and median duration of response of 18.0 months among the 57 total patients with median progression-free survival of 8.5 months. Notably, the PFS of high risk patients was comparable at 8.5 months, driven by the patients with 1q21 gain, characterized by increased MCL-1 expression, with a PFS of 9.1 months versus 3.5 months for the remainder of high risk patients. Patients with t(11;14) also had an encouraging PFS of 15.0 months. The combination of filanesib, bortezomib, and dexamethasone continues to show safety and encouraging activity in relapsed/refractory multiple myeloma, particularly in those patients with 1q21 gain and t(11;14).
Keywords: chemotherapy; clinical cancer research; clinical trials; experimental; medical oncology; multiple myeloma; therapeutics.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Jonathan L. Kaufman has received personal fees and acted as a paid consultant for Amgen, Bristol Meyers Squib, Celgene, Janssen, Sanofi/Genzyme, Takeda, and Tecnopharma and acts as a member of the Advisory Board for Incyte, Karyopharm, Pharmacyclics, and TG Therapeutics. Jeffrey A. Zonder receives research support and personal fees from Celgene and Bristol Meyers Squib and acts as a member of the Advisory Board for Takeda, Amgen, Oncopeptides, Janssen, Intellia, Alnylam, and Caelum. Selena Rush is a former employee of Pfizer and was an employee of Array at the time of the study. Brian J. Tunquist is a current employee of Pfizer and has one approved patent (US Patent 9,561,214) and one patent currently pending (US Patent Application 2018/0338958 [Method of Treatment Using Inhibitors of Mitosis]). Ajai Chari reports grants and personal fees from Janssen, Celgene, Novartis Pharmaceuticals, Seattle Genetics, Millenium/Takeda, and Amgen, personal fees from Bristol Myers Squibb, Karyopharm, Sanofi, Oncopeptides, and Antengene and grants from Pharmacyclics for work performed outside of the current study.
The current study was conducted in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines and regulations. The study was approved by the Institutional Review Boards of all participating centers, and patients provided written informed consent. This study was registered at
Figures
References
-
- Tao W, South VJ, Zhang Y, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49‐59. - PubMed
-
- Tunquist BJ, Woessner RD, Walker DH. Mcl‐1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY‐520. Mol Cancer Ther. 2010;9(7):2046‐2056. - PubMed
-
- Woessner R, Tunquist B, Lemieux C, et al. ARRY‐520, a novel KSP inhibitor with potent activity in hematological and taxane‐resistant tumor models. Anticancer Res. 2009;29:4373‐4380. - PubMed
-
- Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl‐1 rather than Bcl‐2 or Bcl‐xL is an essential survival protein of human myeloma cells. Blood. 2002;100:194‐199. - PubMed

