Influence of breast cancer risk factors on proliferation and DNA damage in human breast glandular tissues: role of intracellular estrogen levels, oxidative stress and estrogen biotransformation
- PMID: 34921608
- PMCID: PMC8837527
- DOI: 10.1007/s00204-021-03198-7
Influence of breast cancer risk factors on proliferation and DNA damage in human breast glandular tissues: role of intracellular estrogen levels, oxidative stress and estrogen biotransformation
Abstract
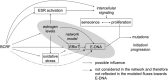
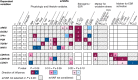
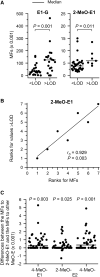
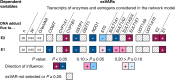
Breast cancer etiology is associated with both proliferation and DNA damage induced by estrogens. Breast cancer risk factors (BCRF) such as body mass index (BMI), smoking, and intake of estrogen-active drugs were recently shown to influence intratissue estrogen levels. Thus, the aim of the present study was to investigate the influence of BCRF on estrogen-induced proliferation and DNA damage in 41 well-characterized breast glandular tissues derived from women without breast cancer. Influence of intramammary estrogen levels and BCRF on estrogen receptor (ESR) activation, ESR-related proliferation (indicated by levels of marker transcripts), oxidative stress (indicated by levels of GCLC transcript and oxidative derivatives of cholesterol), and levels of transcripts encoding enzymes involved in estrogen biotransformation was identified by multiple linear regression models. Metabolic fluxes to adducts of estrogens with DNA (E-DNA) were assessed by a metabolic network model (MNM) which was validated by comparison of calculated fluxes with data on methoxylated and glucuronidated estrogens determined by GC- and UHPLC-MS/MS. Intratissue estrogen levels significantly influenced ESR activation and fluxes to E-DNA within the MNM. Likewise, all BCRF directly and/or indirectly influenced ESR activation, proliferation, and key flux constraints influencing E-DNA (i.e., levels of estrogens, CYP1B1, SULT1A1, SULT1A2, and GSTP1). However, no unambiguous total effect of BCRF on proliferation became apparent. Furthermore, BMI was the only BCRF to indeed influence fluxes to E-DNA (via congruent adverse influence on levels of estrogens, CYP1B1 and SULT1A2).
Keywords: Estrogens; Human breast; Metabolic network model; Multiple linear regression.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Influence of breast cancer risk factors and intramammary biotransformation on estrogen homeostasis in the human breast.Arch Toxicol. 2020 Sep;94(9):3013-3025. doi: 10.1007/s00204-020-02807-1. Epub 2020 Jun 22. Arch Toxicol. 2020. PMID: 32572548 Free PMC article.
-
Qualitative and quantitative differences in estrogen biotransformation in human breast glandular and adipose tissues: implications for studies using mammary biospecimens.Arch Toxicol. 2019 Oct;93(10):2823-2833. doi: 10.1007/s00204-019-02564-w. Epub 2019 Sep 5. Arch Toxicol. 2019. PMID: 31489452
-
Endogenous estrogens as carcinogens through metabolic activation.J Natl Cancer Inst Monogr. 2000;(27):67-73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. J Natl Cancer Inst Monogr. 2000. PMID: 10963620 Review.
-
Modulated expression of genes encoding estrogen metabolizing enzymes by G1-phase cyclin-dependent kinases 6 and 4 in human breast cancer cells.PLoS One. 2014 May 21;9(5):e97448. doi: 10.1371/journal.pone.0097448. eCollection 2014. PLoS One. 2014. PMID: 24848372 Free PMC article.
-
Estrogen metabolism and breast cancer: a risk model.Ann N Y Acad Sci. 2009 Feb;1155:68-75. doi: 10.1111/j.1749-6632.2008.03676.x. Ann N Y Acad Sci. 2009. PMID: 19250193 Free PMC article. Review.
Cited by
-
Association of SULT1A2 rs1059491 with obesity and dyslipidaemia in southern Chinese adults.Sci Rep. 2023 May 4;13(1):7256. doi: 10.1038/s41598-023-34296-4. Sci Rep. 2023. PMID: 37142702 Free PMC article.
-
Human Cytochrome P450 Cancer-Related Metabolic Activities and Gene Polymorphisms: A Review.Cells. 2024 Nov 26;13(23):1958. doi: 10.3390/cells13231958. Cells. 2024. PMID: 39682707 Free PMC article. Review.
-
Antidiabetic effects of Brugmansia aurea leaf extract by modulating the glucose levels, insulin resistance, and oxidative stress mechanism.Front Nutr. 2022 Oct 11;9:1005341. doi: 10.3389/fnut.2022.1005341. eCollection 2022. Front Nutr. 2022. PMID: 36304231 Free PMC article.
References
-
- Buache E, Etique N, Alpy F, Stoll I, Muckensturm M, Reina-San-Martin B, Chenard MP, Tomasetto C, Rio MC. Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice. Oncogene. 2011;30(29):3261–3273. doi: 10.1038/onc.2011.41. - DOI - PMC - PubMed
-
- Cecil A, Rikanovic C, Ohlsen K, Liang C, Bernhardt J, Oelschlaeger TA, Gulder T, Bringmann G, Holzgrabe U, Unger M, Dandekar T. Modeling antibiotic and cytotoxic effects of the dimeric isoquinoline IQ-143 on metabolism and its regulation in Staphylococcus aureus, Staphylococcus epidermidis and human cells. Genome Biol. 2011;12(3):R24. doi: 10.1186/gb-2011-12-3-r24. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

