Effects of amniotic fluid on human keratinocyte gene expression: Implications for wound healing
- PMID: 34921689
- PMCID: PMC9305168
- DOI: 10.1111/exd.14515
Effects of amniotic fluid on human keratinocyte gene expression: Implications for wound healing
Abstract
Cutaneous wounds can lead to huge suffering for patients. Early fetal wounds have the capacity to regenerate without scar formation. Amniotic fluid (AF), containing hyaluronic acid (HA), may contribute to this regenerative environment. We aimed to analyse changes in gene expression when human keratinocytes are exposed to AF or HA. Human keratinocytes were cultured to subconfluence, starved for 12 h and then randomised to be maintained in (1) Dulbecco's modified Eagle's medium (DMEM), (2) DMEM with 50% AF, or (3) DMEM with 50% fetal calf serum (FCS). Transcriptional changes were analysed using microarray and enriched with WebGestalt and Enrichr. Additionally, eight diagnostic genes were analysed using semiquantitative real-time PCR to investigate epidermal differentiation and cellular stress after HA exposure as an alternative for AF exposure. The AF and FCS treatments resulted in enrichment of genes relating to varied aspects of epidermal and keratinocyte biology. In particular, p63-, AP1- and NFE2L2- (Nrf2) associated genes were found significantly regulated in both treatments. More genes regulated by FCS treatment were associated with inflammatory signalling, whilst AF treatment was dominantly associated with molecular establishment of epidermis and lipid metabolic activity. HA exposure mostly resulted in gene regulation that was congruent with the AF microarray group, with increased expression of ITGA6 and LOR. We conclude that AF exposure enhances keratinocyte differentiation in vitro, which suggests that AF constituents can be beneficial for wound-healing applications.
Keywords: PCR; fetal wound healing; human skin cells; in vitro; microarray.
© 2021 The Authors. Experimental Dermatology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

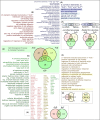
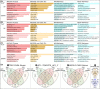

References
-
- Wang PH, Huang BS, Horng HC, Yeh CC, Chen YJ. Wound healing. J Chin Med Assoc. 2018;81(2):94‐101. - PubMed
-
- Gao X, Devoe LD, Given KS. Effects of amniotic fluid on proteases: a possible role of amniotic fluid in fetal wound healing. Ann Plast Surg 1994;33(2):128‐135. discussion 34–5. - PubMed
-
- Sancho MA, Julia V, Albert A, Diaz F, Morales L. Effect of the environment on fetal skin wound healing. J Pediatr Surg. 1997;32(5):663‐666. - PubMed
-
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341‐348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

