Shed CNTNAP2 ectodomain is detectable in CSF and regulates Ca2+ homeostasis and network synchrony via PMCA2/ATP2B2
- PMID: 34921780
- PMCID: PMC8857041
- DOI: 10.1016/j.neuron.2021.11.025
Shed CNTNAP2 ectodomain is detectable in CSF and regulates Ca2+ homeostasis and network synchrony via PMCA2/ATP2B2
Abstract
Although many neuronal membrane proteins undergo proteolytic cleavage, little is known about the biological significance of neuronal ectodomain shedding (ES). Here, we show that the neuronal sheddome is detectable in human cerebrospinal fluid (hCSF) and is enriched in neurodevelopmental disorder (NDD) risk factors. Among shed synaptic proteins is the ectodomain of CNTNAP2 (CNTNAP2-ecto), a prominent NDD risk factor. CNTNAP2 undergoes activity-dependent ES via MMP9 (matrix metalloprotease 9), and CNTNAP2-ecto levels are reduced in the hCSF of individuals with autism spectrum disorder. Using mass spectrometry, we identified the plasma membrane Ca2+ ATPase (PMCA) extrusion pumps as novel CNTNAP2-ecto binding partners. CNTNAP2-ecto enhances the activity of PMCA2 and regulates neuronal network dynamics in a PMCA2-dependent manner. Our data underscore the promise of sheddome analysis in discovering neurobiological mechanisms, provide insight into the biology of ES and its relationship with the CSF, and reveal a mechanism of regulation of Ca2+ homeostasis and neuronal network synchrony by a shed ectodomain.
Keywords: CNTNAP2; autism; bioinformatics; calcium; cerebrospinal fluid; ectodomain shedding; network dynamics; proteomics; schizophrenia; sheddome.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


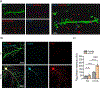
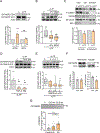

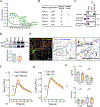
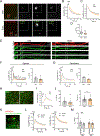

References
-
- Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, et al. (2008). Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet 82, 165–173. 10.1016/j.ajhg.2007.09.017. - DOI - PMC - PubMed
-
- Bayes A, Collins MO, Croning MD, van de Lagemaat LN, Choudhary JS, and Grant SG (2012). Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins. PLoS One 7, e46683. 10.1371/journal.pone.0046683. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

