Efficacy and cost-effectiveness of task-shared care for people with severe mental disorders in Ethiopia (TaSCS): a single-blind, randomised, controlled, phase 3 non-inferiority trial
- PMID: 34921796
- PMCID: PMC8872807
- DOI: 10.1016/S2215-0366(21)00384-9
Efficacy and cost-effectiveness of task-shared care for people with severe mental disorders in Ethiopia (TaSCS): a single-blind, randomised, controlled, phase 3 non-inferiority trial
Abstract
Background: There have been no trials of task-shared care (TSC) using WHO's mental health Gap Action Programme for people with severe mental disorders (psychosis or affective disorder) in low-income or middle-income countries. We aimed to evaluate the efficacy and cost-effectiveness of TSC compared with enhanced specialist mental health care in rural Ethiopia.
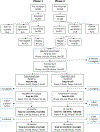
Methods: In this single-blind, phase 3, randomised, controlled, non-inferiority trial, participants had a confirmed diagnosis of a severe mental disorder, recruited from either the community or a local outpatient psychiatric clinic. The intervention was TSC, delivered by supervised, non-physician primary health care workers trained in the mental health Gap Action Programme and working with community health workers. The active comparison group was outpatient psychiatric nurse care augmented with community lay workers (PSY). Our primary endpoint was whether TSC would be non-inferior to PSY at 12 months for the primary outcome of clinical symptom severity using the Brief Psychiatric Rating Scale, Expanded version (BPRS-E; non-inferiority margin of 6 points). Randomisation was stratified by health facility using random permuted blocks. Independent clinicians allocated groups using sealed envelopes with concealment and outcome assessors and investigators were masked. We analysed the primary outcome in the modified intention-to-treat group and safety in the per-protocol group. This trial is registered with ClinicalTrials.gov, number NCT02308956.
Findings: We recruited participants between March 13, 2015 and May 21, 2016. We randomly assigned 329 participants (111 female and 218 male) who were aged 25-72 years and were predominantly of Gurage (198 [60%]), Silte (58 [18%]), and Mareko (53 [16%]) ethnicity. Five participants were found to be ineligible after randomisation, giving a modified intention-to-treat sample of 324. Of these, 12-month assessments were completed in 155 (98%) of 158 in the TSC group and in 158 (95%) of 166 in the PSY group. For the primary outcome, there was no evidence of inferiority of TSC compared with PSY. The mean BPRS-E score was 27·7 (SD 4·7) for TSC and 27·8 (SD 4·6) for PSY, with an adjusted mean difference of 0·06 (90% CI -0·80 to 0·89). Per-protocol analyses (n=291) were similar. There were 47 serious adverse events (18 in the TSC group, 29 in the PSY group), affecting 28 participants. These included 17 episodes of perpetrated violence and seven episodes of violent victimisation leading to injury, ten suicide attempts, six hospital admissions for physical health conditions, four psychiatric admissions, and three deaths (one in the TSC group, two in the PSY group). The incremental cost-effectiveness ratio for TSC indicated lower cost of -US$299·82 (95% CI -454·95 to -144·69) per unit increase in BPRS-E scores from a health care sector perspective at 12 months.
Interpretation: WHO's mental health Gap Action Programme for people with severe mental disorders is as cost-effective as existing specialist models of care and can be implemented effectively and safely by supervised non-specialists in resource-poor settings.
Funding: US National Institute of Mental Health.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Quality care for people with severe mental disorders in low-resource settings.Lancet Psychiatry. 2022 Jan;9(1):3-5. doi: 10.1016/S2215-0366(21)00438-7. Lancet Psychiatry. 2022. PMID: 34921794 No abstract available.
References
-
- Fekadu A, Medhin G, Kebede D, et al. Excess mortality in severe mental disorders: a 10-year population-based cohort study in rural Ethiopia. British Journal of Psychiatry 2015; 206(4): 289–96. - PubMed
-
- Drew N, Funk M, Tang S, et al. Human rights violations of people with mental and psychosocial disabilities: an unresolved global crisis. Lancet (London, England) 2011; 378(9803): 1664–75. - PubMed
-
- World Health Organization. mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings: mental health Gap Action Programme (mhGAP). http://www.who.int/mental_health/evidence/mhGAP_intervention_guide/en/in.... Geneva: WHO, 2010. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous