A limited access oral oxycodone paradigm produces physical dependence and mesocorticolimbic region-dependent increases in DeltaFosB expression without preference
- PMID: 34921830
- PMCID: PMC8817207
- DOI: 10.1016/j.neuropharm.2021.108925
A limited access oral oxycodone paradigm produces physical dependence and mesocorticolimbic region-dependent increases in DeltaFosB expression without preference
Abstract
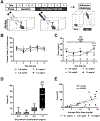
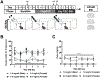
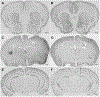
The abuse of oral formulations of prescription opioids has precipitated the current opioid epidemic. We developed an oral oxycodone consumption model consisting of a limited access (4 h) two-bottle choice drinking in the dark (TBC-DID) paradigm and quantified dependence with naloxone challenge using mice of both sexes. We also assessed neurobiological correlates of withdrawal and dependence elicited via oral oxycodone consumption using immunohistochemistry for DeltaFosB (ΔFosB), a transcription factor described as a molecular marker for drug addiction. Neither sex developed a preference for the oxycodone bottle, irrespective of oxycodone concentration, bottle position or prior water restriction. Mice that volitionally consumed oxycodone exhibited hyperlocomotion in an open field test and supraspinal but not spinally-mediated antinociception. Both sexes also developed robust, dose-dependent levels of opioid withdrawal that was precipitated by the opioid antagonist naloxone. Oral oxycodone consumption followed by naloxone challenge led to mesocorticolimbic region-dependent increases in the number of ΔFosB expressing cells. Naloxone-precipitated withdrawal jumps, but not the oxycodone bottle % preference, was positively correlated with the number of ΔFosB expressing cells specifically in the nucleus accumbens shell. Thus, limited access oral consumption of oxycodone produced physical dependence and increased ΔFosB expression despite the absence of opioid preference. Our TBC-DID paradigm allows for the study of oral opioid consumption in a simple, high-throughput manner and elucidates the underlying neurobiological substrates that accompany opioid-induced physical dependence.
Keywords: Dependence; Mouse; Oral self-administration; Oxycodone; Prescription opioid; Withdrawal.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Figures


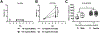


Similar articles
-
Comparing withdrawal- and anxiety-like behaviors following oral and subcutaneous oxycodone administration in C57BL/6 mice.Behav Pharmacol. 2024 Aug 1;35(5):269-279. doi: 10.1097/FBP.0000000000000780. Epub 2024 Jun 3. Behav Pharmacol. 2024. PMID: 38847447 Free PMC article.
-
Validation and characterization of oxycodone physical dependence in C57BL/6J mice.Eur J Pharmacol. 2021 Jul 15;903:174111. doi: 10.1016/j.ejphar.2021.174111. Epub 2021 Apr 23. Eur J Pharmacol. 2021. PMID: 33901461
-
Effects of CB1 receptor negative allosteric modulator Org27569 on oxycodone withdrawal symptoms in mice.Psychopharmacology (Berl). 2024 Aug;241(8):1705-1717. doi: 10.1007/s00213-024-06591-z. Epub 2024 Apr 27. Psychopharmacology (Berl). 2024. PMID: 38676755 Free PMC article.
-
Oxycodone/Naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation.Drugs. 2014 Mar;74(3):353-75. doi: 10.1007/s40265-014-0177-9. Drugs. 2014. PMID: 24452879 Review.
-
Elucidating the molecular symphony: unweaving the transcriptional & epigenetic pathways underlying neuroplasticity in opioid dependence and withdrawal.Psychopharmacology (Berl). 2024 Oct;241(10):1955-1981. doi: 10.1007/s00213-024-06684-9. Epub 2024 Sep 10. Psychopharmacology (Berl). 2024. PMID: 39254835 Review.
Cited by
-
Investigating the potential of mirtazapine to induce drug-seeking behavior in free-choice drinking mouse model.Saudi Pharm J. 2022 Dec;30(12):1809-1815. doi: 10.1016/j.jsps.2022.10.010. Epub 2022 Nov 2. Saudi Pharm J. 2022. PMID: 36601513 Free PMC article.
-
An ethogram analysis of cutaneous thermal pain sensitivity and oxycodone reward-related behaviors in rats.Sci Rep. 2023 Jun 28;13(1):10482. doi: 10.1038/s41598-023-36729-6. Sci Rep. 2023. PMID: 37380739 Free PMC article.
-
Comparing withdrawal- and anxiety-like behaviors following oral and subcutaneous oxycodone administration in C57BL/6 mice.Behav Pharmacol. 2024 Aug 1;35(5):269-279. doi: 10.1097/FBP.0000000000000780. Epub 2024 Jun 3. Behav Pharmacol. 2024. PMID: 38847447 Free PMC article.
-
Negative allosteric modulation of CB1 cannabinoid receptor signaling suppresses opioid-mediated tolerance and withdrawal without blocking opioid antinociception.Neuropharmacology. 2024 Oct 1;257:110052. doi: 10.1016/j.neuropharm.2024.110052. Epub 2024 Jun 25. Neuropharmacology. 2024. PMID: 38936657 Free PMC article.
-
Mice lacking the endocannabinoid-synthesizing enzyme NAPE-PLD exhibit sex-dependent dysregulations in responsiveness to oxycodone and a natural reward.Neuropharmacology. 2025 Nov 1;278:110573. doi: 10.1016/j.neuropharm.2025.110573. Epub 2025 Jun 26. Neuropharmacology. 2025. PMID: 40581209
References
-
- Ahmad F, Rossen L, Sutton P, 2021. Provisional drug overdose death counts National Center for Health Statistics. 2021. Statistics, Centers for Disease Control and Prevention (CDC).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

