Histogram analysis of tensor-valued diffusion MRI in meningiomas: Relation to consistency, histological grade and type
- PMID: 34922122
- PMCID: PMC8688887
- DOI: 10.1016/j.nicl.2021.102912
Histogram analysis of tensor-valued diffusion MRI in meningiomas: Relation to consistency, histological grade and type
Abstract
Background: Preoperative radiological assessment of meningioma characteristics is of value for pre- and post-operative patient management, counselling, and surgical approach.
Purpose: To investigate whether tensor-valued diffusion MRI can add to the preoperative prediction of meningioma consistency, grade and type.
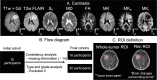
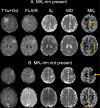
Materials and methods: 30 patients with intracranial meningiomas (22 WHO grade I, 8 WHO grade II) underwent MRI prior to surgery. Diffusion MRI was performed with linear and spherical b-tensors with b-values up to 2000 s/mm2. The data were used to estimate mean diffusivity (MD), fractional anisotropy (FA), mean kurtosis (MK) and its components-the anisotropic and isotropic kurtoses (MKA and MKI). Meningioma consistency was estimated for 16 patients during resection based on ultrasonic aspiration intensity, ease of resection with instrumentation or suction. Grade and type were determined by histopathological analysis. The relation between consistency, grade and type and dMRI parameters was analyzed inside the tumor ("whole-tumor") and within brain tissue in the immediate periphery outside the tumor ("rim") by histogram analysis.
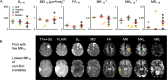
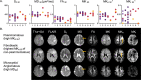
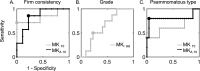
Results: Lower 10th percentiles of MK and MKA in the whole-tumor were associated with firm consistency compared with pooled soft and variable consistency (n = 7 vs 9; U test, p = 0.02 for MKA 10 and p = 0.04 for MK10) and lower 10th percentile of MD with variable against soft and firm (n = 5 vs 11; U test, p = 0.02). Higher standard deviation of MKI in the rim was associated with lower grade (n = 22 vs 8; U test, p = 0.04) and in the MKI maps we observed elevated rim-like structure that could be associated with grade. Higher median MKA and lower median MKI distinguished psammomatous type from other pooled meningioma types (n = 5 vs 25; U test; p = 0.03 for MKA 50 and p = 0.03 and p = 0.04 for MKI 50).
Conclusion: Parameters from tensor-valued dMRI can facilitate prediction of consistency, grade and type.
Keywords: Consistency; Diffusion MRI; Meningioma; Tensor-valued diffusion encoding; Tumor grade; Type.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M.N. declares ownership interests in Random Walk Imaging, and patent applications in Sweden (1250453-6 and 1250452-8), in the USA (61/642 594 and 61/642 589), and via the Patent Cooperation Treaty (SE2013/050492 and SE2013/050493). M.N. and F.S. are inventors on pending patents pertaining to the methods presented herein. None of the other authors have any conflict of interest to disclose. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


References
-
- Bi W.L., Prabhu V.C., Dunn I.F. High-grade meningiomas: biology and implications. Neurosurg. Focus. 2018;44:E2. - PubMed
-
- Cepeda S., Arrese I., García-García S., Velasco-Casares M., Escudero-Caro T., Zamora T., Sarabia R. Meningioma consistency can be defined by combining the radiomic features of magnetic resonance imaging and ultrasound elastography. A pilot study using machine learning classifiers. World Neurosurg. 2021;146:e1147–e1159. - PubMed
-
- Chartrain A.G., Kurt M., Yao A., Feng R., Nael K., Mocco J., Bederson J.B., Balchandani P., Shrivastava R.K. Utility of preoperative meningioma consistency measurement with magnetic resonance elastography (MRE): a review. Neurosurg. Rev. 2019;42:1–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

