Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study
- PMID: 34922444
- PMCID: PMC8903705
- DOI: 10.1186/s10194-021-01363-y
Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study
Abstract
Background: Monoclonal antibodies anti-calcitonin gene-related peptide (mAbs anti-CGRP) pathway are effective and safe on migraine prevention. However, some drug agencies limited these treatments to one year due to their high costs. This study aimed at evaluating the effect of discontinuing mAbs anti-CGRP on monthly migraine days (MMDs) and disability in high-frequency episodic (HFEM) and chronic migraine (CM) patients.
Methods: This observational longitudinal cohort study was conducted at 10 Italian headache centres. Consecutive adult patients were followed-up for three months (F-UP1-3) after discontinuation of a one-year erenumab/galcanezumab treatment. The primary endpoint was the change in F-UP MMDs. Secondary endpoints included variation in pain intensity (Numerical Rating Scale, NRS), monthly acute medication intake (MAMI), and HIT-6 scores. We also assessed from F-UP1 to 3 the ≥50% response rate, relapse rate to CM, and recurrence of Medication Overuse (MO).
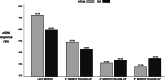
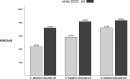
Results: We enrolled 154 patients (72.1% female, 48.2 ± 11.1 years, 107 CM, 47 HFEM); 91 were treated with erenumab, 63 with galcanezumab. From F-UP1 to F-UP3, MMDs, MAMI, NRS, and HIT-6 progressively increased but were still lower at F-UP3 than baseline (Friedman's analysis of rank, p < .001). In the F-UP1-3 visits, ≥50% response rate frequency did not differ significantly between CM and HFEM patients. However, the median reduction in response rate at F-UP3 was higher in HFEM (- 47.7% [25th, - 79.5; 75th,-17.0]) than in CM patients (- 25.5% [25th, - 47.1; 75th, - 3.3]; Mann-Whitney U test; p = .032). Of the 84 baseline CM patients who had reverted to episodic migraine, 28 (33.3%) relapsed to CM at F-UP1, 35 (41.7%) at F-UP2, 39 (46.4%) at F-UP3. Of the 64 baseline patients suffering of medication overuse headache ceasing MO, 15 (18.3%) relapsed to MO at F-UP1, 26 (31.6%) at F-UP2, and 30 (42.3%, 11 missing data) at F-UP3. Lower MMDs, MAMI, NRS, and HIT-6 and higher response rate in the last month of therapy characterized patients with ≥50% response rate at F-UP1 and F-UP3 (Mann-Whitney U test; consistently p < .01).
Conclusion: Migraine frequency and disability gradually increased after mAbs anti-CGRP interruption. Most patients did not relapse to MO or CM despite the increase in MMDs. Our data suggest to reconsider mAbs anti-CGRP discontinuation.
Keywords: Calcitonin gene-related peptide; Discontinuation; Migraine treatment; Monoclonal antibodies; Real-world.
© 2021. The Author(s).
Conflict of interest statement
Fabrizio Vernieri received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan, Amgen, Angelini, Eli-Lilly, Lundbeck, Novartis, and Teva.
Roberta Messina received honoraria as speaker from Novartis, Eli Lilly, and Teva.
Bruno Colombo received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Novartis, Teva, Eli-Lilly Lusofarmaco Paola Torelli received travel grant, honoraria as a speaker, or for participating in advisory boards from Novartis, Teva, Eli Lilly, and Allergan.
Sabina Cevoli received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Novartis, Teva, Lilly, Allergan, Ibsa, Amgen and Lundbeck.
Valentina Favoni received honoraria as speaker or for participating in advisory boards from Ely-Lilly, Novartis and Teva.
Florindo d’Onofrio received grants and honoraria from Lilly, Teva, Novartis, Neopharmed.
Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta and Ecupharma.
Renata Rao received honoraria for speaker panels from Teva, Lilly, Novartis.
Massimo Filippi is the Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and AriSLA (Fondazione Italiana di Ricerca per la SLA).
Piero Barbanti received travel grants, honoraria for advisory boards, speaker panels or clinical investigation studies from Alder, Allergan, Angelini, Bayer, ElectroCore, Eli-Lilly, GSK, Lusofarmaco, MSD, Novartis, Stx-Med, Teva, Visufarma, Zambon.
Claudia Altamura received travel grants and honoraria from Novartis, Eli Lilly, Lusofarmaco, Laborest, Allergan, Almirall.
Nicoletta Brunelli, Simone Quintana and Carmelina Maria Costa have nothing to disclose.
Figures


Similar articles
-
Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study).J Headache Pain. 2021 May 3;22(1):35. doi: 10.1186/s10194-021-01247-1. J Headache Pain. 2021. PMID: 33941080 Free PMC article.
-
Impact of multiple treatment cycles with anti-CGRP monoclonal antibodies on migraine course: focus on discontinuation periods. Insights from the multicenter, prospective, I-GRAINE study.J Neurol. 2024 May;271(5):2605-2614. doi: 10.1007/s00415-024-12192-9. Epub 2024 Feb 12. J Neurol. 2024. PMID: 38342785 Free PMC article.
-
Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: Results of the EARLY 2 study.Headache. 2021 Oct;61(9):1351-1363. doi: 10.1111/head.14194. Epub 2021 Jul 26. Headache. 2021. PMID: 34309862
-
The transition of medication overuse status by acute medication categories in episodic or chronic migraine patients to non-overuse status after receiving anti-CGRP monoclonal antibodies: a systematic review and meta-analysis of phase 3 randomized control trial.Neurol Sci. 2024 Sep;45(9):4451-4462. doi: 10.1007/s10072-024-07496-7. Epub 2024 Apr 2. Neurol Sci. 2024. PMID: 38564060
-
Safety, efficacy, and compliance of moderate-to-high dose eptinezumab and erenumab in chronic migraine patients with medication-overuse headache: an updated systematic review and meta-analysis.J Headache Pain. 2025 May 6;26(1):99. doi: 10.1186/s10194-025-02047-7. J Headache Pain. 2025. PMID: 40329188 Free PMC article.
Cited by
-
Enhancing Acute Migraine Treatment: Exploring Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for the Nose-to-Brain Route.Pharmaceutics. 2024 Oct 4;16(10):1297. doi: 10.3390/pharmaceutics16101297. Pharmaceutics. 2024. PMID: 39458626 Free PMC article. Review.
-
Real-world evidence following a mandatory treatment break after a 1-year prophylactic treatment with calcitonin gene-related peptide (pathway) monoclonal antibodies.Brain Behav. 2022 Jul;12(7):e2662. doi: 10.1002/brb3.2662. Epub 2022 Jun 10. Brain Behav. 2022. PMID: 35687795 Free PMC article.
-
The effect of erenumab on brain network function in episodic migraine patients: a randomized, placebo-controlled clinical trial (RESET BRAIN).J Neurol. 2023 Nov;270(11):5600-5612. doi: 10.1007/s00415-023-11879-9. Epub 2023 Aug 8. J Neurol. 2023. PMID: 37550498 Free PMC article. Clinical Trial.
-
CROATIAN GUIDELINES FOR SPECIFIC PREVENTIVE TREATMENT OF MIGRAINE WITH MONOCLONAL ANTIBODIES TARGETING CALCITONIN GENE-RELATED PEPTIDE (CGRP) (EPTINEZUMAB, FREMANEZUMAB, AND GALCANEZUMAB) OR THE CGRP RECEPTOR (ERENUMAB).Acta Clin Croat. 2024 Oct;63(2):436-450. doi: 10.20471/acc.2024.63.02.22. Acta Clin Croat. 2024. PMID: 40104234 Free PMC article. Review.
-
Methodological considerations on real-world evidence studies of monoclonal antibodies against the CGRP-pathway for migraine: a systematic review.J Headache Pain. 2023 Jun 22;24(1):75. doi: 10.1186/s10194-023-01611-3. J Headache Pain. 2023. PMID: 37344811 Free PMC article.
References
-
- Edvinsson L, Haanes KA, Warfvinge K, DiN K (2018) CGRP as the target of new migraine therapies - Successful translation from bench to clinic. Nat Rev Neurol 14:338–350 - PubMed
-
- Tepper S, Ashina M, Reuter U, Brandes JL, Doležil D, Silberstein S, Winner P, Leonardi D, Mikol D, Lenz R. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434. doi: 10.1016/S1474-4422(17)30083-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

