The automation of relevant trial registration screening for systematic review updates: an evaluation study on a large dataset of ClinicalTrials.gov registrations
- PMID: 34922458
- PMCID: PMC8684229
- DOI: 10.1186/s12874-021-01485-6
The automation of relevant trial registration screening for systematic review updates: an evaluation study on a large dataset of ClinicalTrials.gov registrations
Abstract
Background: Clinical trial registries can be used as sources of clinical evidence for systematic review synthesis and updating. Our aim was to evaluate methods for identifying clinical trial registrations that should be screened for inclusion in updates of published systematic reviews.
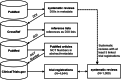
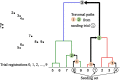
Methods: A set of 4644 clinical trial registrations (ClinicalTrials.gov) included in 1089 systematic reviews (PubMed) were used to evaluate two methods (document similarity and hierarchical clustering) and representations (L2-normalised TF-IDF, Latent Dirichlet Allocation, and Doc2Vec) for ranking 163,501 completed clinical trials by relevance. Clinical trial registrations were ranked for each systematic review using seeding clinical trials, simulating how new relevant clinical trials could be automatically identified for an update. Performance was measured by the number of clinical trials that need to be screened to identify all relevant clinical trials.
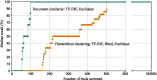
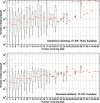
Results: Using the document similarity method with TF-IDF feature representation and Euclidean distance metric, all relevant clinical trials for half of the systematic reviews were identified after screening 99 trials (IQR 19 to 491). The best-performing hierarchical clustering was using Ward agglomerative clustering (with TF-IDF representation and Euclidean distance) and needed to screen 501 clinical trials (IQR 43 to 4363) to achieve the same result.
Conclusion: An evaluation using a large set of mined links between published systematic reviews and clinical trial registrations showed that document similarity outperformed hierarchical clustering for identifying relevant clinical trials to include in systematic review updates.
Keywords: Document similarity; Hierarchical clustering; Systematic reviews; Trial registrations.
© 2021. The Author(s).
Conflict of interest statement
None.
Figures
Similar articles
-
A comparison of machine learning methods to find clinical trials for inclusion in new systematic reviews from their PROSPERO registrations prior to searching and screening.Res Synth Methods. 2024 Jan;15(1):73-85. doi: 10.1002/jrsm.1672. Epub 2023 Sep 25. Res Synth Methods. 2024. PMID: 37749068 Free PMC article.
-
A shared latent space matrix factorisation method for recommending new trial evidence for systematic review updates.J Biomed Inform. 2018 Mar;79:32-40. doi: 10.1016/j.jbi.2018.01.008. Epub 2018 Feb 2. J Biomed Inform. 2018. PMID: 29410356
-
Unreported links between trial registrations and published articles were identified using document similarity measures in a cross-sectional analysis of ClinicalTrials.gov.J Clin Epidemiol. 2018 Mar;95:94-101. doi: 10.1016/j.jclinepi.2017.12.007. Epub 2017 Dec 19. J Clin Epidemiol. 2018. PMID: 29277557
-
Reliability of Trial Information Across Registries for Trials With Multiple Registrations: A Systematic Review.JAMA Netw Open. 2021 Nov 1;4(11):e2128898. doi: 10.1001/jamanetworkopen.2021.28898. JAMA Netw Open. 2021. PMID: 34724557 Free PMC article.
-
A systematic review of comparisons between protocols or registrations and full reports in primary biomedical research.BMC Med Res Methodol. 2018 Jan 11;18(1):9. doi: 10.1186/s12874-017-0465-7. BMC Med Res Methodol. 2018. PMID: 29325533 Free PMC article.
Cited by
-
Automation of systematic reviews of biomedical literature: a scoping review of studies indexed in PubMed.Syst Rev. 2024 Jul 8;13(1):174. doi: 10.1186/s13643-024-02592-3. Syst Rev. 2024. PMID: 38978132 Free PMC article.
-
A comparison of machine learning methods to find clinical trials for inclusion in new systematic reviews from their PROSPERO registrations prior to searching and screening.Res Synth Methods. 2024 Jan;15(1):73-85. doi: 10.1002/jrsm.1672. Epub 2023 Sep 25. Res Synth Methods. 2024. PMID: 37749068 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

