Nanofactory for metabolic and chemodynamic therapy: pro-tumor lactate trapping and anti-tumor ROS transition
- PMID: 34922541
- PMCID: PMC8684183
- DOI: 10.1186/s12951-021-01169-9
Nanofactory for metabolic and chemodynamic therapy: pro-tumor lactate trapping and anti-tumor ROS transition
Abstract
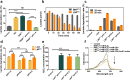
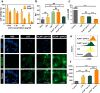
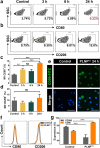
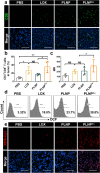
Lactate plays a critical role in tumorigenesis, invasion and metastasis. Exhausting lactate in tumors holds great promise for the reversal of the immunosuppressive tumor microenvironment (TME). Herein, we report on a "lactate treatment plant" (i.e., nanofactory) that can dynamically trap pro-tumor lactate and in situ transformation into anti-tumor cytotoxic reactive oxygen species (ROS) for a synergistic chemodynamic and metabolic therapy. To this end, lactate oxidase (LOX) was nano-packaged by cationic polyethyleneimine (PEI), assisted by a necessary amount of copper ions (PLNPCu). As a reservoir of LOX, the tailored system can actively trap lactate through the cationic PEI component to promote lactate degradation by two-fold efficiency. More importantly, the byproducts of lactate degradation, hydrogen peroxide (H2O2), can be transformed into anti-tumor ROS catalyzing by copper ions, mediating an immunogenic cell death (ICD). With the remission of immunosuppressive TME, ICD process effectively initiated the positive immune response in 4T1 tumor model (88% tumor inhibition). This work provides a novel strategy that rationally integrates metabolic therapy and chemodynamic therapy (CDT) for combating tumors.
Keywords: Chemodynamic therapy; Immunogenic cell death; Immunosuppressive tumor microenvironment; Lactate.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
A ROS storm generating nanocomposite for enhanced chemodynamic therapy through H2O2 self-supply, GSH depletion and calcium overload.Nanoscale. 2024 May 2;16(17):8479-8494. doi: 10.1039/d3nr06422k. Nanoscale. 2024. PMID: 38590261
-
Copper-doped layered double hydroxides co-deliver proteins/drugs for cascaded chemodynamic/immunotherapy via dual regulation of tumor metabolism.Acta Biomater. 2025 Mar 15;195:350-362. doi: 10.1016/j.actbio.2025.02.008. Epub 2025 Feb 5. Acta Biomater. 2025. PMID: 39921184
-
Sono-Triggered Cascade Lactate Depletion by Semiconducting Polymer Nanoreactors for Cuproptosis-Immunotherapy of Pancreatic Cancer.Angew Chem Int Ed Engl. 2024 Jul 22;63(30):e202405639. doi: 10.1002/anie.202405639. Epub 2024 Jun 17. Angew Chem Int Ed Engl. 2024. PMID: 38708791
-
Harnessing inorganic nanomaterials for chemodynamic cancer therapy.Nanomedicine (Lond). 2022 Oct;17(24):1891-1906. doi: 10.2217/nnm-2022-0187. Epub 2023 Jan 17. Nanomedicine (Lond). 2022. PMID: 36647807 Review.
-
Recent progress in lactate oxidase-based drug delivery systems for enhanced cancer therapy.Nanoscale. 2024 May 9;16(18):8739-8758. doi: 10.1039/d3nr05952a. Nanoscale. 2024. PMID: 38602362 Review.
Cited by
-
Applications and enhancement strategies of ROS-based non-invasive therapies in cancer treatment.Redox Biol. 2025 Mar;80:103515. doi: 10.1016/j.redox.2025.103515. Epub 2025 Jan 28. Redox Biol. 2025. PMID: 39904189 Free PMC article. Review.
-
Nanomaterials-driven in situ vaccination: a novel frontier in tumor immunotherapy.J Hematol Oncol. 2025 Apr 17;18(1):45. doi: 10.1186/s13045-025-01692-4. J Hematol Oncol. 2025. PMID: 40247328 Free PMC article. Review.
-
Antiviral Activity of Marine Bacterium Paraliobacillus zengyii Against Enterovirus 71 In Vitro and In Vivo.Int J Mol Sci. 2025 Apr 8;26(8):3500. doi: 10.3390/ijms26083500. Int J Mol Sci. 2025. PMID: 40331950 Free PMC article.
-
Mechanism and application of copper-based nanomedicines in activating tumor immunity through oxidative stress modulation.Front Pharmacol. 2025 Jul 11;16:1646890. doi: 10.3389/fphar.2025.1646890. eCollection 2025. Front Pharmacol. 2025. PMID: 40717985 Free PMC article. Review.
-
Nanomaterials: leading immunogenic cell death-based cancer therapies.Front Immunol. 2024 Aug 9;15:1447817. doi: 10.3389/fimmu.2024.1447817. eCollection 2024. Front Immunol. 2024. PMID: 39185425 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

