1,3,4-Oxadiazole-naphthalene hybrids as potential VEGFR-2 inhibitors: design, synthesis, antiproliferative activity, apoptotic effect, and in silico studies
- PMID: 34923885
- PMCID: PMC8725909
- DOI: 10.1080/14756366.2021.2015342
1,3,4-Oxadiazole-naphthalene hybrids as potential VEGFR-2 inhibitors: design, synthesis, antiproliferative activity, apoptotic effect, and in silico studies
Erratum in
-
Correction.J Enzyme Inhib Med Chem. 2022 Dec;37(1):514. doi: 10.1080/14756366.2022.2024999. J Enzyme Inhib Med Chem. 2022. PMID: 34986713 Free PMC article. No abstract available.
Abstract
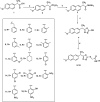
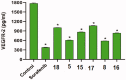
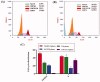
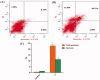
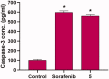
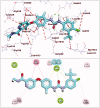
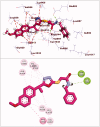
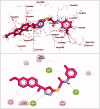
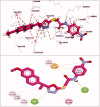
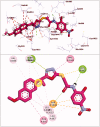
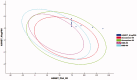
In the current work, some 1,3,4-oxadiazole-naphthalene hybrids were designed and synthesised as VEGFR-2 inhibitors. The synthesised compounds were evaluated in vitro for their antiproliferative activity against two human cancer cell lines namely, HepG-2 and MCF-7. Compounds that exhibited promising cytotoxicity (5, 8, 15, 16, 17, and 18) were further evaluated for their VEGFR-2 inhibitory activities. Compound 5 showed good antiproliferative activity against both cell lines and inhibitory effect on VEGFR-2. Besides, it induced apoptosis by 22.86% compared to 0.51% in the control (HepG2) cells. This apoptotic effect was supported by a 5.61-fold increase in the level of caspase-3 compared to the control cells. Moreover, it arrested the HepG2 cell growth mostly at the Pre-G1 phase. Several in silico studies were performed including docking, ADMET, and toxicity studies to predict binding mode against VEGFR-2 and to anticipate pharmacokinetic, drug-likeness, and toxicity of the synthesised compounds.
Keywords: 1,3,4-oxadiazole; Anticancer; VEGFR-2 inhibitors; apoptosis; docking.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- WHO, Cancer. https://www.who.int/health-topics/cancer#tab=tab_1. (Accessed May 2020 2020).
-
- Thurston DE. Chemistry and pharmacology of anticancer drugs. 2nd ed. London: CRC Press, Tylor and Francis; 2006.
-
- Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E.. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis 2018;35:309–18. - PubMed
-
- El-Zahabi MA, Sakr H, El-Adl K, et al. . Design, synthesis, and biological evaluation of new challenging thalidomide analogs as potential anticancer immunomodulatory agents. Bioorg Chem 2020;104:104218. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
