Flavonoids as tyrosinase inhibitors in in silico and in vitro models: basic framework of SAR using a statistical modelling approach
- PMID: 34923888
- PMCID: PMC8735877
- DOI: 10.1080/14756366.2021.2014832
Flavonoids as tyrosinase inhibitors in in silico and in vitro models: basic framework of SAR using a statistical modelling approach
Erratum in
-
Correction.J Enzyme Inhib Med Chem. 2022 Dec;37(1):514. doi: 10.1080/14756366.2022.2024999. J Enzyme Inhib Med Chem. 2022. PMID: 34986713 Free PMC article. No abstract available.
Abstract
Flavonoids are widely distributed in plants and constitute the most common polyphenolic phytoconstituents in the human diet. In this study, the in vitro inhibitory activity of 44 different flavonoids (1-44) against mushroom tyrosinase was studied, and an in silico study and type of inhibition for the most active compounds were evaluated too. Tyrosinase inhibitors block melanogenesis and take part in melanin production or distribution leading to pigmentation diseases. The in vitro study showed that quercetin was a competitive inhibitor (IC50=44.38 ± 0.13 µM) and achieved higher antityrosinase activity than the control inhibitor kojic acid. The in silico results highlight the importance of the flavonoid core with a hydroxyl at C7 as a strong contributor of interference with tyrosinase activity. According to the developed statistical model, the activity of molecules depends on hydroxylation at C3 and methylation at C8, C7, and C3 in the benzo-γ-pyrane ring of the flavonoids.
Keywords: Flavonoid; molecular docking; statistical analysis; structure-activity relationship; tyrosinase.
Conflict of interest statement
No potential conflict of interest was reported by the authors. CT Supuran is Editor-in-Chief of Journal of Enzyme Inhibition and Medicinal Chemistry and he was not involved in the assessment, peer review or decision making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
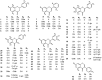
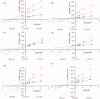

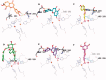
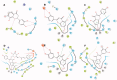
Comment in
-
Oxidation of flavonoids by tyrosinase and by o-quinones-comment on "Flavonoids as tyrosinase inhibitors in in silico and in vitro models: basic framework of SAR using a statistical modelling approach" published by K. Jakimiuk, S. Sari, R. Milewski, C.T. Supuran, D. Şöhretoğlu, and M. Tomczyk (J Enzyme Inhib Med Chem 2022;37:427-436).J Enzyme Inhib Med Chem. 2023 Dec;38(1):2269611. doi: 10.1080/14756366.2023.2269611. Epub 2023 Oct 16. J Enzyme Inhib Med Chem. 2023. PMID: 37842733 Free PMC article. No abstract available.
References
-
- Jakimiuk K, Wink M, Tomczyk M.. Flavonoids of the Caryophyllaceae. Phytochem Rev 2021;20:1–40.
-
- García-Sosa AR, Maran U.. Improving the use of ranking in virtual screening against HIV-1 integrase with triangular numbers and including ligand profiling with antitargets. J Chem Inf Model 2014;54:3172–85. - PubMed
-
- Flurkey WH, Inlow JK.. Proteolytic processing of polyphenol oxidase from plants and fungi. J Inorg Biochem 2008;102:2160–70. - PubMed
-
- Ismaya WT, Rozeboom HJ, Weijn A, et al. . Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochem 2011;50:5477–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
