Structure and function of ClpXP, a AAA+ proteolytic machine powered by probabilistic ATP hydrolysis
- PMID: 34923891
- PMCID: PMC9871882
- DOI: 10.1080/10409238.2021.1979461
Structure and function of ClpXP, a AAA+ proteolytic machine powered by probabilistic ATP hydrolysis
Abstract
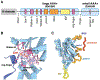
ClpXP is an archetypical AAA+ protease, consisting of ClpX and ClpP. ClpX is an ATP-dependent protein unfoldase and polypeptide translocase, whereas ClpP is a self-compartmentalized peptidase. ClpXP is currently the only AAA+ protease for which high-resolution structures exist, the molecular basis of recognition for a protein substrate is understood, extensive biochemical and genetic analysis have been performed, and single-molecule optical trapping has allowed direct visualization of the kinetics of substrate unfolding and translocation. In this review, we discuss our current understanding of ClpXP structure and function, evaluate competing sequential and probabilistic mechanisms of ATP hydrolysis, and highlight open questions for future exploration.
Keywords: AAA+ proteases; ATP hydrolysis; ClpP; ClpX; cryo-EM; molecular machine; protein degradation.
Conflict of interest statement
Disclosure statement
The authors declare no conflict of interest.
Figures
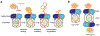
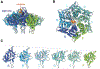

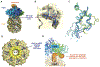


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
