Insights into the post-translational modification and its emerging role in shaping the tumor microenvironment
- PMID: 34924561
- PMCID: PMC8685280
- DOI: 10.1038/s41392-021-00825-8
Insights into the post-translational modification and its emerging role in shaping the tumor microenvironment
Erratum in
-
Correction: Insights into the post-translational modification and its emerging role in shaping the tumor microenvironment.Signal Transduct Target Ther. 2022 Jan 31;7(1):31. doi: 10.1038/s41392-022-00901-7. Signal Transduct Target Ther. 2022. PMID: 35095101 Free PMC article. No abstract available.
Abstract
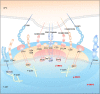
More and more in-depth studies have revealed that the occurrence and development of tumors depend on gene mutation and tumor heterogeneity. The most important manifestation of tumor heterogeneity is the dynamic change of tumor microenvironment (TME) heterogeneity. This depends not only on the tumor cells themselves in the microenvironment where the infiltrating immune cells and matrix together forming an antitumor and/or pro-tumor network. TME has resulted in novel therapeutic interventions as a place beyond tumor beds. The malignant cancer cells, tumor infiltrate immune cells, angiogenic vascular cells, lymphatic endothelial cells, cancer-associated fibroblastic cells, and the released factors including intracellular metabolites, hormonal signals and inflammatory mediators all contribute actively to cancer progression. Protein post-translational modification (PTM) is often regarded as a degradative mechanism in protein destruction or turnover to maintain physiological homeostasis. Advances in quantitative transcriptomics, proteomics, and nuclease-based gene editing are now paving the global ways for exploring PTMs. In this review, we focus on recent developments in the PTM area and speculate on their importance as a critical functional readout for the regulation of TME. A wealth of information has been emerging to prove useful in the search for conventional therapies and the development of global therapeutic strategies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing of interests.
Figures


References
-
- Corner BM, Cawley PF. Vibrio parahaemolyticus-food poisoning: case report. N. Z. Med. J. 1976;83:155–156. - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Dittmer J, Leyh B. The impact of tumor stroma on drug response in breast cancer. Semin. Cancer Biol. 2015;31:3–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

