Induction of cell apoptosis by biliverdin reductase inhibitor in MCF-7 and MDA-MB-468 breast cancer cell lines: Experimental and in silico studies
- PMID: 34924900
- PMCID: PMC8678058
- DOI: 10.17179/excli2021-4069
Induction of cell apoptosis by biliverdin reductase inhibitor in MCF-7 and MDA-MB-468 breast cancer cell lines: Experimental and in silico studies
Abstract
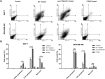
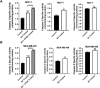
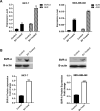
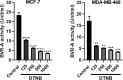
Biliverdin reductase, biliverdin and bilirubin are known as important components of cellular signaling pathways that play major roles in cell proliferation and apoptosis, although their physiological relevance is still under evaluation. This study was designed to investigate the expression and activity of BVR-A and its apoptotic effect in the breast cancer cell lines, MCF-7 and MDA-MB-468. The expression of BVR-A was examined by real-time PCR and western blot analysis. Bilirubin concentration was measured by HPLC and molecular docking was performed to identify an appropriate inhibitor for BVR-A. To detect cell apoptosis, annexin V-PI staining, caspase-3, -8, and -9 activities were evaluated. Cell viability was reduced by biliverdin, in a dose-dependent manner, and an intrinsic apoptotic response occurred which was evidenced by caspase-3 and -9 activities. The intra- and extracellular concentrations of bilirubin were higher in MCF-7 cells than those of MDA-MB-468 cells. The expression of BVR-A, at mRNA and protein levels, in MCF-7 was also higher than that of MDA-MB-468 cells. Treatment of both cell lines with biliverdin plus DTNB, a BVR-A inhibitor, increased the cell death significantly when compared with biliverdin alone. Using annexin V-PI staining and assessment of caspase-3 activity, it was confirmed that biliverdin together with DTNB increases apoptosis in breast cancer cells. In conclusion, biliverdin has an important role in cell apoptosis and inhibition of biliverdin reductase increases the apoptotic effect of biliverdin.
Keywords: biliverdin reductase-A; breast cancer cell lines; caspase activity; high-performance liquid chromatography; molecular docking.
Copyright © 2021 Shahrokhi et al.
Figures





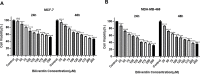

Similar articles
-
Cellular effect of styrene substituted biscoumarin caused cellular apoptosis and cell cycle arrest in human breast cancer cells.Int J Biochem Cell Biol. 2017 Nov;92:104-114. doi: 10.1016/j.biocel.2017.09.019. Epub 2017 Sep 25. Int J Biochem Cell Biol. 2017. PMID: 28958615
-
Biliverdin reductase/bilirubin mediates the anti-apoptotic effect of hypoxia in pulmonary arterial smooth muscle cells through ERK1/2 pathway.Exp Cell Res. 2013 Aug 1;319(13):1973-1987. doi: 10.1016/j.yexcr.2013.05.015. Epub 2013 May 28. Exp Cell Res. 2013. PMID: 23722043
-
Activation of Intrinsic Apoptosis and G1 Cell Cycle Arrest by a Triazole Precursor, N-(4-chlorophenyl)-2-(4-(3,4,5-trimethoxybenzyloxy)benzoyl)-hydrazinecarbothioamide in Breast Cancer Cell Line.Anticancer Agents Med Chem. 2020;20(9):1072-1086. doi: 10.2174/1871520620666200318100051. Anticancer Agents Med Chem. 2020. PMID: 32188392
-
Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance.Pharmacol Rep. 2008 Jan-Feb;60(1):38-48. Pharmacol Rep. 2008. PMID: 18276984 Free PMC article. Review.
-
Biliverdin reductase: a target for cancer therapy?Front Pharmacol. 2015 Jun 3;6:119. doi: 10.3389/fphar.2015.00119. eCollection 2015. Front Pharmacol. 2015. PMID: 26089799 Free PMC article. Review.
Cited by
-
5-Aminolevrinic Acid Exhibits Dual Effects on Stemness in Human Sarcoma Cell Lines under Dark Conditions.Int J Mol Sci. 2023 Mar 24;24(7):6189. doi: 10.3390/ijms24076189. Int J Mol Sci. 2023. PMID: 37047157 Free PMC article.
-
Molecular mechanisms of bilirubin induced G1 cell cycle arrest and apoptosis in human breast cancer cell lines: involvement of the intrinsic pathway.Mol Biol Rep. 2022 Nov;49(11):10421-10429. doi: 10.1007/s11033-022-07757-8. Epub 2022 Sep 14. Mol Biol Rep. 2022. PMID: 36104587
References
-
- Arena V, Pennacchia I, Guerriero G, Mancuso C. The heme oxygenase/biliverdin reductase system in skin cancers. J Biol Regul Homeost Agents. 2015;29:259–264. - PubMed
-
- Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, et al. Biliverdin reductase--a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta. 2011;1812:480–487. doi: 10.1016/j.bbadis.2011.01.005. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
