Physalin A Inhibits MAPK and NF-κB Signal Transduction Through Integrin αVβ3 and Exerts Chondroprotective Effect
- PMID: 34925020
- PMCID: PMC8678602
- DOI: 10.3389/fphar.2021.761922
Physalin A Inhibits MAPK and NF-κB Signal Transduction Through Integrin αVβ3 and Exerts Chondroprotective Effect
Abstract
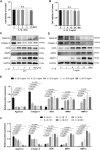
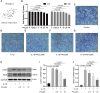
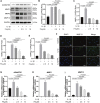
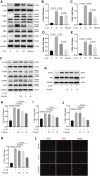
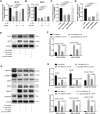
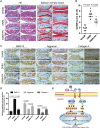
Osteoarthritis (OA) is a common articular ailment presented with cartilage loss and destruction that is common observed in the elderly population. Physalin A (PA), a natural bioactive withanolide, exerts anti-inflammatory residences in more than a few diseases; however, little is known about its efficacy for OA treatment. Here, we explored the therapeutic effects and potential mechanism of PA in mouse OA. After the in vitro administration of PA, the expression of inflammation indicators including inducible nitric oxide synthase and cyclooxygenase-2 was low, indicating that PA could alleviate the IL-1β-induced chondrocyte inflammation response. Moreover, PA reduced IL-1β-induced destruction of the extracellular matrix by upregulating the gene expression of anabolism factors, including collagen II, aggrecan, and sry-box transcription factor 9, and downregulating the gene expression of catabolic factors, including thrombospondin motif 5 and matrix metalloproteinases. In addition, the chondroprotective effect of PA was credited to the inhibition of mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways. Furthermore, in vivo experiments showed that intra-articular injection of PA could alleviate cartilage destruction in a mouse OA model. However, the anti-inflammatory, anabolism enhancing, catabolism inhibiting, and MAPK and NF-κB signaling pathway inhibiting properties of PA on IL-1β-induced chondrocytes could be reversed when integrin αVβ3 is knocked down by siRNA. In conclusion, our work demonstrates that PA exhibits a chondroprotective effect that may be mediated by integrin αVβ3. Thus, PA or integrin αVβ3 might be a promising agent or molecular target for the treatment of OA.
Keywords: MAPK; NF-κB; integrin; osteoarthritis; physalin A; αvβ3.
Copyright © 2021 Lu, Yu, Liang, Cheng, Wang, He, Lv, Wan, Mo, Zhu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Attur M. G., Dave M. N., Clancy R. M., Patel I. R., Abramson S. B., Amin A. R. (2000). Functional Genomic Analysis in Arthritis-Affected Cartilage: Yin-Yang Regulation of Inflammatory Mediators by Alpha 5 Beta 1 and Alpha V Beta 3 Integrins. J. Immunol. 164 (5), 2684–2691. 10.4049/jimmunol.164.5.2684 - DOI - PubMed
-
- Buckwalter J. A., Mankin H. J., Grodzinsky A. J. (2005). Articular Cartilage and Osteoarthritis. Instr. Course Lect 54, 465–480. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

