Elder Mice Exhibit More Severe Degeneration and Milder Regeneration in Temporomandibular Joints Subjected to Bilateral Anterior Crossbite
- PMID: 34925057
- PMCID: PMC8674645
- DOI: 10.3389/fphys.2021.750468
Elder Mice Exhibit More Severe Degeneration and Milder Regeneration in Temporomandibular Joints Subjected to Bilateral Anterior Crossbite
Abstract
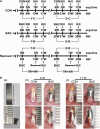
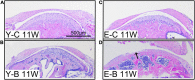
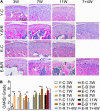
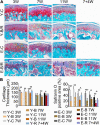
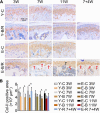
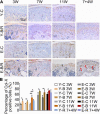
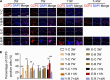
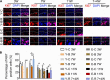
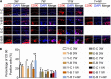
Temporomandibular joints (TMJs) have a biomechanical relationship with dental occlusion. Aberrant occlusion initiates degenerative remodeling responses in TMJ condyles. Aging is a promoting factor of osteoarthritis (OA) development. The aim of this study was to assess the effect of aging on degenerative remodeling in TMJ condyles in response to occlusal biomechanical stimulation caused by the installation of aberrant prostheses and observe rehabilitation after their removal. The experiments involved 84 female C57BL/6J mice (42 at 6 weeks old and 42 at 28 weeks old). A bilateral anterior crossbite (BAC) model was developed, and the TMJs were sampled at 3, 7, and 11 weeks. BAC was removed at 7 weeks in a subset of mice, which accepted BAC treatment at 6 week of age, and maintained for another 4 weeks after BAC removal. TMJ changes were assessed with micro-CT, histomorphology, immunohistochemistry (IHC), and immunofluorescence staining assays. The results showed that BAC induced typical OA-like TMJ lesions that were more severe in the elder groups as evaluated by the acellular zones, clustered chondrocytes, fissures between cartilage and subchondral bone, reductions in matrix amount and the cartilage thickness as revealed by histomorphological measurements, and subchondral bone loss as detected on micro-CT images. IHC indicated significant increases in cleaved caspase-3-expressing cells and decreases in ki67-positive cells in the BAC groups. There were obvious age-dependent changes in the numbers of superficial zone cells and CD90-expressing cells. Supportively, cleaved caspase-3-expressing cells obviously increased, while ki67-expressing cells significantly decreased with aging. In the elder BAC groups, the superficial zone cells such as CD90-expressing cells were greatly reduced. At 11 weeks, the superficial zone cells were almost non-existent, and there were clear serrated injuries on the cartilage surface. BAC removal attenuated the degenerative changes in the condylar cartilage and subchondral bone. Notably, the rescue effect was more pronounced in the younger animals. Our findings demonstrate the impacts of aging on both TMJ degenerative changes in response to BAC and regenerative changes following BAC removal. The reduced number of chondro-progenitor cells in aged TMJ cartilage provides an explanation for this age-related decline in TMJ rehabilitative behaviors.
Keywords: aging; cartilage; occlusion; subchondral bone; temporomandibular joint.
Copyright © 2021 Zhang, Xu, Zhou, Liu, Zhang, Yang, Yu, Zhang, Huo, Zhao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Installing and thereafter removing an aberrant prosthesis elicited opposite remodelling responses in growing mouse temporomandibular joints.J Oral Rehabil. 2015 Sep;42(9):685-92. doi: 10.1111/joor.12304. Epub 2015 May 1. J Oral Rehabil. 2015. PMID: 25940877
-
Temporomandibular joint disc responses to installation and removal of the experimental malocclusion.J Oral Rehabil. 2024 Dec;51(12):2503-2512. doi: 10.1111/joor.13833. Epub 2024 Aug 12. J Oral Rehabil. 2024. PMID: 39135294
-
Reducing dietary loading decreases mouse temporomandibular joint degradation induced by anterior crossbite prosthesis.Osteoarthritis Cartilage. 2014 Feb;22(2):302-12. doi: 10.1016/j.joca.2013.11.014. Epub 2013 Dec 5. Osteoarthritis Cartilage. 2014. PMID: 24316289 Free PMC article.
-
Initiation and progression of dental-stimulated temporomandibular joints osteoarthritis.Osteoarthritis Cartilage. 2021 May;29(5):633-642. doi: 10.1016/j.joca.2020.12.016. Epub 2021 Jan 8. Osteoarthritis Cartilage. 2021. PMID: 33422706 Review.
-
Different effects of abnormal mechanical stress on temporomandibular joint cartilage, subchondral bone, and discs.Front Physiol. 2025 Apr 3;16:1539342. doi: 10.3389/fphys.2025.1539342. eCollection 2025. Front Physiol. 2025. PMID: 40247923 Free PMC article. Review.
Cited by
-
Practice and challenge of age-friendly functional restoration of stomatognathic system based on the strategy of functional tooth loss.Hua Xi Kou Qiang Yi Xue Za Zhi. 2025 Feb 1;43(1):15-27. doi: 10.7518/hxkq.2025.2024188. Hua Xi Kou Qiang Yi Xue Za Zhi. 2025. PMID: 39840622 Free PMC article. Chinese, English.
-
Heterogeneous Characteristics of the CD90+ Progenitors in the Fibrocartilage of Different Joints.Cartilage. 2025 Jun;16(2):190-201. doi: 10.1177/19476035231200359. Epub 2023 Sep 26. Cartilage. 2025. PMID: 37750508 Free PMC article.
-
Global Trends and Future Research Directions for Temporomandibular Disorders and Stem Cells.J Funct Biomater. 2023 Feb 13;14(2):103. doi: 10.3390/jfb14020103. J Funct Biomater. 2023. PMID: 36826902 Free PMC article. Review.
-
Zonal interdependence in the temporomandibular joint cartilage.FASEB J. 2023 Apr;37(4):e22888. doi: 10.1096/fj.202201662RR. FASEB J. 2023. PMID: 36961420 Free PMC article.
-
Animal Models of Temporomandibular Joint Osteoarthritis: Classification and Selection.Front Physiol. 2022 Apr 28;13:859517. doi: 10.3389/fphys.2022.859517. eCollection 2022. Front Physiol. 2022. PMID: 35574432 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

