Early Hyperdynamic Sepsis Alters Coronary Blood Flow Regulation in Porcine Fecal Peritonitis
- PMID: 34925058
- PMCID: PMC8678271
- DOI: 10.3389/fphys.2021.754570
Early Hyperdynamic Sepsis Alters Coronary Blood Flow Regulation in Porcine Fecal Peritonitis
Abstract
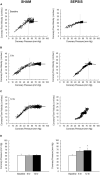
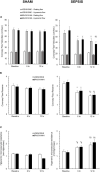
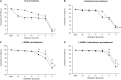
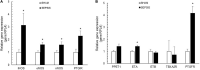
Background: Sepsis is a common condition known to impair blood flow regulation and microcirculation, which can ultimately lead to organ dysfunction but such contribution of the coronary circulation remains to be clarified. We investigated coronary blood flow regulatory mechanisms, including autoregulation, metabolic regulation, and endothelial vasodilatory response, in an experimental porcine model of early hyperdynamic sepsis. Methods: Fourteen pigs were randomized to sham (n = 7) or fecal peritonitis-induced sepsis (n = 7) procedures. At baseline, 6 and 12 h after peritonitis induction, the animals underwent general and coronary hemodynamic evaluation, including determination of autoregulatory breakpoint pressure and adenosine-induced maximal coronary vasodilation for coronary flow reserve and hyperemic microvascular resistance calculation. Endothelial-derived vasodilatory response was assessed both in vivo and ex vivo using bradykinin. Coronary arteries were sampled for pathobiological evaluation. Results: Sepsis resulted in a right shift of the autoregulatory breakpoint pressure, decreased coronary blood flow reserve and increased hyperemic microvascular resistance from the 6th h after peritonitis induction. In vivo and ex vivo endothelial vasomotor function was preserved. Sepsis increased coronary arteries expressions of nitric oxide synthases, prostaglandin I2 receptor, and prostaglandin F2α receptor. Conclusion: Autoregulation and metabolic blood flow regulation were both impaired in the coronary circulation during experimental hyperdynamic sepsis, although endothelial vasodilatory response was preserved.
Keywords: autoregulation; coronary blood flow; endothelial function; metabolic regulation; microcirculation; sepsis.
Copyright © 2021 Boudart, Su, Pitisci, Dhoine, Duranteau, Jespers, Herpain, Vanderpool, Brimioulle, Creteur, Naeije, Van Obbergh and Dewachter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bimodal Pattern of Coronary Microvascular Involvement in Diabetes Mellitus.J Am Heart Assoc. 2016 Nov 14;5(11):e003995. doi: 10.1161/JAHA.116.003995. J Am Heart Assoc. 2016. PMID: 27930353 Free PMC article.
-
Regulation of coronary blood flow during exercise.Physiol Rev. 2008 Jul;88(3):1009-86. doi: 10.1152/physrev.00045.2006. Physiol Rev. 2008. PMID: 18626066 Review.
-
Vital organ blood flow during hyperdynamic sepsis.Chest. 2003 Sep;124(3):1053-9. doi: 10.1378/chest.124.3.1053. Chest. 2003. PMID: 12970037
-
Contribution of Age-Related Microvascular Dysfunction to Abnormal Coronary: Hemodynamics in Patients With Ischemic Heart Disease.JACC Cardiovasc Interv. 2020 Jan 13;13(1):20-29. doi: 10.1016/j.jcin.2019.08.052. JACC Cardiovasc Interv. 2020. PMID: 31918939
-
Coronary microcirculation: autoregulation and metabolic control.Basic Res Cardiol. 1995 Mar-Apr;90(2):112-8. doi: 10.1007/BF00789441. Basic Res Cardiol. 1995. PMID: 7646415 Review.
Cited by
-
Septic Cardiomyopathy: Difficult Definition, Challenging Diagnosis, Unclear Treatment.J Clin Med. 2025 Feb 4;14(3):986. doi: 10.3390/jcm14030986. J Clin Med. 2025. PMID: 39941657 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

