Differences in Endothelial Activation and Dysfunction Induced by Antiphospholipid Antibodies Among Groups of Patients With Thrombotic, Refractory, and Non-refractory Antiphospholipid Syndrome
- PMID: 34925061
- PMCID: PMC8675389
- DOI: 10.3389/fphys.2021.764702
Differences in Endothelial Activation and Dysfunction Induced by Antiphospholipid Antibodies Among Groups of Patients With Thrombotic, Refractory, and Non-refractory Antiphospholipid Syndrome
Abstract
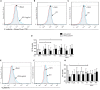
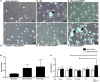
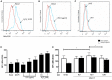
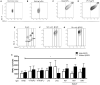
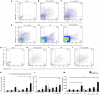
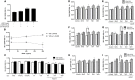
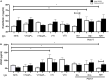
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by pregnancy morbidity or thrombosis and persistent antiphospholipid antibodies (aPL) that bind to the endothelium and induce endothelial activation, which is evidenced by the expression of adhesion molecules and the production of reactive oxygen species (ROS) and subsequent endothelial dysfunction marked by a decrease in the synthesis and release of nitric oxide (NO). These endothelial alterations are the key components for the development of severe pathological processes in APS. Patients with APS can be grouped according to the presence of other autoimmune diseases (secondary APS), thrombosis alone (thrombotic APS), pregnancy morbidity (obstetric APS), and refractoriness to conventional treatment regimens (refractory APS). Typically, patients with severe and refractory obstetric APS exhibit thrombosis and are classified as those having primary or secondary APS. The elucidation of the mechanisms underlying these alterations according to the different groups of patients with APS could help establish new therapies, particularly necessary for severe and refractory cases. Therefore, this study aimed to evaluate the differences in endothelial activation and dysfunction induced by aPL between patients with refractory obstetric APS and other APS clinical manifestations. Human umbilical vein endothelial cells (HUVECs) were stimulated with polyclonal immunoglobulin-G (IgG) from different groups of patients n = 21), including those with primary (VTI) and secondary thrombotic APS (VTII) and refractory primary (RI+), refractory secondary (RII+), and non-refractory primary (NR+) obstetric APS. All of them with thrombosis. The expression of adhesion molecules; the production of ROS, NO, vascular endothelial growth factor (VEGF), and endothelin-1; and the generation of microparticles were used to evaluate endothelial activation and dysfunction. VTI IgG induced the expression of adhesion molecules and the generation of microparticles and VEGF. RI+ IgG induced the expression of adhesion molecules and decreased NO production. RII+ IgG increased the production of microparticles, ROS, and endothelin-1 and reduced NO release. NR+ IgG increased the production of microparticles and endothelin-1 and decreased the production of VEGF and NO. These findings reveal differences in endothelial activation and dysfunction among groups of patients with APS, which should be considered in future studies to evaluate new therapies, especially in refractory cases.
Keywords: antiphosholipid syndrome; antiphospholipid syndrome; beta 2-glycoprotein I; endothelial activation and dysfunction; endothelial cells; immunoglobulin G.
Copyright © 2021 Velásquez, Peláez, Rojas, Narváez-Sánchez, Velásquez, Escudero, San Martín and Cadavid.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Antiphospholipid Antibodies From Women With Pregnancy Morbidity and Vascular Thrombosis Induce Endothelial Mitochondrial Dysfunction, mTOR Activation, and Autophagy.Front Physiol. 2021 Nov 29;12:706743. doi: 10.3389/fphys.2021.706743. eCollection 2021. Front Physiol. 2021. PMID: 34912234 Free PMC article.
-
Mechanisms of Endothelial Dysfunction in Antiphospholipid Syndrome: Association With Clinical Manifestations.Front Physiol. 2018 Dec 21;9:1840. doi: 10.3389/fphys.2018.01840. eCollection 2018. Front Physiol. 2018. PMID: 30627104 Free PMC article. Review.
-
Added value of non-criteria antiphospholipid antibodies for antiphospholipid syndrome: lessons learned from year-long routine measurements.Clin Rheumatol. 2019 Feb;38(2):371-378. doi: 10.1007/s10067-018-4251-7. Epub 2018 Aug 11. Clin Rheumatol. 2019. PMID: 30099654
-
Modulation of the activation of endothelial nitric oxide synthase and nitrosative stress biomarkers by aspirin triggered lipoxins: A possible mechanism of action of aspirin in the antiphospholipid syndrome.Am J Reprod Immunol. 2023 Aug;90(2):e13753. doi: 10.1111/aji.13753. Am J Reprod Immunol. 2023. PMID: 37491919
-
Probing antiphospholipid-mediated thrombosis: the interplay between anticardiolipin antibodies and endothelial cells.Lupus. 2003;12(7):539-45. doi: 10.1191/961203303lu398oa. Lupus. 2003. PMID: 12892395 Review.
Cited by
-
Role of Lipid Rafts on LRP8 Signaling Triggered by Anti-β2-GPI Antibodies in Endothelial Cells.Biomedicines. 2023 Nov 24;11(12):3135. doi: 10.3390/biomedicines11123135. Biomedicines. 2023. PMID: 38137358 Free PMC article.
-
Longitudinal assessment of cerebral infarcts and small vessel disease using magnetic resonance imaging in antiphospholipid syndrome: A single-centre retrospective study.EJHaem. 2025 Feb 6;6(1):e1065. doi: 10.1002/jha2.1065. eCollection 2025 Feb. EJHaem. 2025. PMID: 39917355 Free PMC article.
-
Can complement activation be the missing link in antiphospholipid syndrome?Rheumatology (Oxford). 2024 Dec 1;63(12):3243-3254. doi: 10.1093/rheumatology/keae178. Rheumatology (Oxford). 2024. PMID: 38483257 Free PMC article. Review.
-
The effects of hydroxychloroquine and its promising use in refractory obstetric antiphospholipid syndrome.Rheumatol Int. 2024 Feb;44(2):223-234. doi: 10.1007/s00296-023-05457-5. Epub 2023 Sep 23. Rheumatol Int. 2024. PMID: 37741812 Free PMC article. Review.
-
In Vitro Assessment of Poly-N-Vinylpyrrolidone/Acrylic Acid Nanoparticles Biocompatibility in a Microvascular Endothelium Model.Int J Mol Sci. 2022 Oct 18;23(20):12446. doi: 10.3390/ijms232012446. Int J Mol Sci. 2022. PMID: 36293301 Free PMC article.
References
-
- Alvarez A. M., Balcazar N., San Martin S., Markert U. R., Cadavid A. P. (2017). Modulation of antiphospholipid antibodies-induced trophoblast damage by different drugs used to prevent pregnancy morbidity associated with antiphospholipid syndrome. Am. J. Reprod. Immunol. 77:e12634. 10.1111/aji.12634 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

