Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation
- PMID: 34925360
- PMCID: PMC8674945
- DOI: 10.3389/fimmu.2021.780641
Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation
Abstract
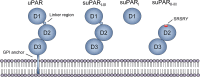
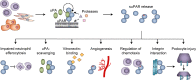
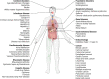
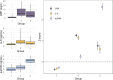
Systemic chronic inflammation (SCI) is persistent, health-damaging, low-grade inflammation that plays a major role in immunosenescence and in development and progression of many diseases. But currently, there are no recognized standard biomarkers to assess SCI levels alone, and SCI is typically measured by combining biomarkers of acute inflammation and infection, e.g., CRP, IL-6, and TNFα. In this review, we highlight 10 properties and characteristics that are shared by the blood protein soluble urokinase plasminogen activator receptor (suPAR) and SCI, supporting the argument that suPAR is a biomarker of SCI: (1) Expression and release of suPAR is upregulated by immune activation; (2) uPAR and suPAR exert pro-inflammatory functions; (3) suPAR is associated with the amount of circulating immune cells; (4) Blood suPAR levels correlate with the levels of established inflammatory biomarkers; (5) suPAR is minimally affected by acute changes and short-term influences, in contrast to many currently used markers of systemic inflammation; (6) Like SCI, suPAR is non-specifically associated with multiple diseases; (7) suPAR and SCI both predict morbidity and mortality; (8) suPAR and SCI share the same risk factors; (9) suPAR is associated with risk factors and outcomes of inflammation above and beyond other inflammatory biomarkers; (10) The suPAR level can be reduced by anti-inflammatory interventions and treatment of disease. Assessing SCI has the potential to inform risk for morbidity and mortality. Blood suPAR is a newer biomarker which may, in fact, be a biomarker of SCI since it is stably associated with inflammation and immune activation; shares the same risk factors as many age-related diseases; is both elevated by and predicts age-related diseases. There is strong evidence that suPAR is a prognostic marker of adverse events, morbidity, and mortality. It is associated with immune activity and prognosis across diverse conditions, including kidney disease, cardiovascular disease, cancer, diabetes, and inflammatory disorders. Thus, we think it likely represents a common underlying disease-process shared by many diseases; that is, SCI. We review the supporting literature and propose a research agenda that can help test the hypothesis that suPAR indexes SCI, with the potential of becoming the new gold standard for measuring SCI.
Keywords: C-reactive protein; biomarkers; immunosenescence; inflammaging; inflammation; inflammation mediators - blood; interleukin-6.
Copyright © 2021 Rasmussen, Petersen and Eugen-Olsen.
Conflict of interest statement
JE-O is a named inventor on patents on suPAR as a prognostic biomarker. The patents are owned by Copenhagen University Hospital Amager and Hvidovre, Denmark, and is licensed to ViroGates A/S. JE-O is a co-founder, shareholder, and CSO of ViroGates A/S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization . (2014). Global Status Report on Noncommunicable Diseases 2014. World Health Organization. https://apps.who.int/iris/handle/10665/148114
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

