Immune Metabolism of IL-4-Activated B Cells and Th2 Cells in the Context of Allergic Diseases
- PMID: 34925372
- PMCID: PMC8671807
- DOI: 10.3389/fimmu.2021.790658
Immune Metabolism of IL-4-Activated B Cells and Th2 Cells in the Context of Allergic Diseases
Abstract
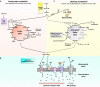
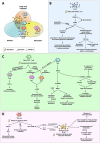
Over the last decades, the frequency of allergic disorders has steadily increased. Immunologically, allergies are caused by abnormal immune responses directed against otherwise harmless antigens derived from our environment. Two of the main cell types driving allergic sensitization and inflammation are IgE-producing plasma cells and Th2 cells. The acute activation of T and B cells, their differentiation into effector cells, as well as the formation of immunological memory are paralleled by distinct changes in cellular metabolism. Understanding the functional consequences of these metabolic changes is the focus of a new research field termed "immune metabolism". Currently, the contribution of metabolic changes in T and B cells to either the development or maintenance of allergies is not completely understood. Therefore, this mini review will introduce the fundamentals of energy metabolism, its connection to immune metabolism, and subsequently focus on the metabolic phenotypes of IL-4-activated B cells and Th2 cells.
Keywords: Warburg metabolism; allergies; fatty acid oxidation (FAO); glycolysis; immune metabolism; metabolism; oxidative phosphorylation.
Copyright © 2021 Lin, Goretzki and Schülke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

