Dunalialla salina microalgea and its isolated zeaxanthin mitigate age-related dementia in rats: Modulation of neurotransmission and amyloid-β protein
- PMID: 34926168
- PMCID: PMC8648797
- DOI: 10.1016/j.toxrep.2021.11.021
Dunalialla salina microalgea and its isolated zeaxanthin mitigate age-related dementia in rats: Modulation of neurotransmission and amyloid-β protein
Abstract
Age-related deterioration of sensorimotor and cognitive abilities suggests that the brain undergoes regressive alterations with aging that compromise its function. Thus, the present study was designed to assess the efficacy of Dunaliella salina in counteracting D-galactose (D-gal)-induced dementia brain aging and its modulatory role in attenuating amyloid β (Aβ) protein and neurotransmitters. Aging associated dementia was generated by injection of D-gal (200 mg/kg; i.p) of rats for 8 weeks. D. salina biomass (250 mg/kg), polar (30 mg/kg), its carotenoid (30 mg/kg) fractions as well as the isolated zeaxanthin (250 μg/kg) were given orally simultaneously with D-gal for additional two weeks. Twenty-four hours after the last treatment dose; behavioral, biochemical and histopathological assessment were performed. Results showed that oral treatment of motor deficit rats with D. salina biomass and its isolated polar and carotenoid fractions showed amelioration in the motor coordination assessed by the rotarod test and in the memory and learning capabilities evaluated by Morris water maze test. D. salina also showed a reduction in brain levels of inflammatory indicators viz. interlekin-1β and inducible nitric oxide synthetase as well as brain contents of Aβ protein and myelin base protein. Likewise, oral treatment with D. salina biomass and its isolated polar and carotenoid fractions exhibited an increase in the rats' brain neurotransmitters and their metabolites. Furthermore, histopathological investigations have confirmed all of these results. Our findings suggest that D. salina overcomes brain aging and thereby repairs age-related dementia, both for its modulating function in attenuating the Aβ protein and neurotransmitters.
Keywords: Aging; Amyloid-β protein; Dementia; Dunalialla salina; Neurotransmitters.
© 2021 The Author(s).
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

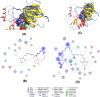

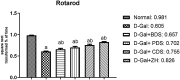

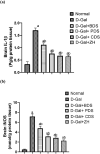
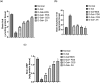


References
-
- Aloizou A.-M., Siokas V., Vogiatzi C., Peristeri E., Docea A.O., Petrakis D., et al. Pesticides, cognitive functions and dementia: a review. Toxicol. Lett. 2020;326:31–51. - PubMed
-
- Gubandru M., Margina D., Tsitsimpikou C., Goutzourelas N., Tsarouhas K., Ilie M., et al. Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food and Chem. Toxicol.: an Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;61:209–214. - PubMed
LinkOut - more resources
Full Text Sources

