Photoredox Chemistry with Organic Catalysts: Role of Computational Methods
- PMID: 34926877
- PMCID: PMC8674904
- DOI: 10.1021/acsomega.1c05787
Photoredox Chemistry with Organic Catalysts: Role of Computational Methods
Abstract
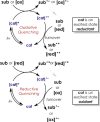
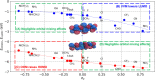
Organic catalysts have the potential to carry out a wide range of otherwise thermally inaccessible reactions via photoredox routes. Early demonstrated successes of organic photoredox catalysts include one-electron CO2 reduction and H2 generation via water splitting. Photoredox systems are challenging to study and design owing to the sheer number and diversity of phenomena involved, including light absorption, emission, intersystem crossing, partial or complete charge transfer, and bond breaking or formation. Designing a viable photoredox route therefore requires consideration of a host of factors such as absorption wavelength, solvent, choice of electron donor or acceptor, and so on. Quantum chemistry methods can play a critical role in demystifying photoredox phenomena. Using one-electron CO2 reduction with phenylene-based chromophores as an illustrative example, this perspective highlights recent developments in quantum chemistry that can advance our understanding of photoredox processes and proposes a way forward for driving the design and discovery of organic catalysts.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

