Humoral Responses After SARS-CoV-2 mRNA Vaccination and Breakthrough Infection in Cancer Patients
- PMID: 34926993
- PMCID: PMC8666324
- DOI: 10.1016/j.mayocpiqo.2021.12.004
Humoral Responses After SARS-CoV-2 mRNA Vaccination and Breakthrough Infection in Cancer Patients
Abstract
Objective: To evaluate the magnitude of humoral response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines in patients with cancer receiving active therapies.
Patients and methods: Patients 18 years or older in whom SARS-CoV-2 spike antibody (anti-S Ab) levels were measured after 2 doses of SARS-CoV-2 mRNA vaccines were included. Patients with prior coronavirus disease 2019 (COVID-19) infection or receiving other immunosuppressive therapy were excluded.
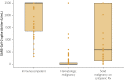
Results: Among 201 patients who met the criteria, 61 were immunocompetent, 91 had a hematologic malignancy, and 49 had a solid malignancy while receiving treatments associated with cytopenia, including chemotherapy or cyclin-dependent kinase 4 and 6 inhibitors. A significantly greater proportion of immunocompetent patients (96.7% [59 of 61]) had anti-S Ab titers of 500 U/mL or greater compared to patients with hematologic (7.7% [7 of 91) and solid (55.1% [27 of 49]) malignancy (P<.001). Despite 2 doses of SARS-CoV-2 mRNA vaccines, 52.7% of patients with hematologic malignancy (48 of 91) and 8.2% of those with solid malignancy (4 of 49) receiving cytopenic therapy had no seroconversion (spike antibody titers <0.8 U/mL). Two patients subsequently had development of breakthrough COVID-19 infection after full vaccination.
Conclusion: A substantial proportion of patients with hematologic and solid malignancies receiving chemotherapies and CDK4/6i had poor humoral responses after SARS-CoV-2 mRNA vaccination. Our study adds to a growing body of literature suggesting that immunosuppressed patients have a suboptimal humoral response to COVID-19 vaccination. Our study also underscores the importance of assessing antibody response after COVID-19 vaccines in these vulnerable patients.
Keywords: Anti-S Ab, SARS-CoV-2 spike antibody; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor(s); CLL, chronic lymphocytic leukemia; COVID-19, coronavirus disease 2019; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; mRNA, messenger RNA.
© 2021 The Authors.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

