The hyoid as a sound conducting apparatus in laryngeally echolocating bats
- PMID: 34927244
- PMCID: PMC9119617
- DOI: 10.1111/joa.13615
The hyoid as a sound conducting apparatus in laryngeally echolocating bats
Abstract
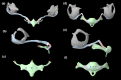
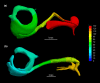
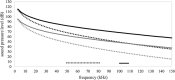
The morphology of the stylohyal-tympanic bone articulation found in laryngeally echolocating bats is highly indicative of a function associated with signal production. One untested hypothesis is that this morphology allows the transfer of a sound signal from the larynx to the tympanic bones (auditory bulla) via the hyoid apparatus during signal production by the larynx. We used µCT data and finite element analysis to model the propagation of sound through the hyoid chain into the tympanic bones to test this hypothesis. We modeled sound pressure (dB) wave propagation from the basihyal to the tympanic bones, vibratory behavior (m) of the stylohyal-tympanic bone unit, and the stylohyal and tympanic bones when the stylohyal bone is allowed to pivot on the tympanic bone. Sound pressure wave propagation was modeled using the harmonic acoustics solver in ANSYS and vibratory behavior was modeled using coupled modal and harmonic response analyses in ANSYS. For both analyses (harmonic acoustics and harmonic response), the input excitation on the basihyal and thyrohyals was modeled as the estimated pressure (Pa) imposed by the collision of the vibrating thyroid cartilage of the larynx against these bones during signal production. Our models support the hypothesis that this stereotypical hyoid morphology found in laryngeally echolocating bats can transfer sound to the auditory bullae at an amplitude that is likely heard for the species Artibeus jamaicensis and Rhinolophus pusillus.
Keywords: Chiroptera; bioacoustics; biomechanics; sonar.
© 2021 Anatomical Society.
Figures


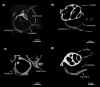
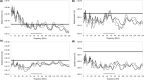
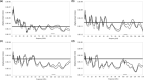
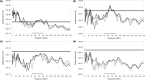

References
-
- Brinkløv, S. , Kalko, E.K. & Surlykke, A. (2009) Intense echolocation calls from two ‘whispering’ bats, Artibeus jamaicensis and Macrophyllum macrophyllum (Phyllostomidae). Journal of Experimental Biology, 212, 11–20. - PubMed
-
- Carter, R.T. & Adams, R.A. (2014) Ontogeny of the larynx and flight ability in Jamaican fruit bats (Phyllostomidae) with considerations for the evolution of echolocation. The Anatomical Record, 297, 1270–1277. - PubMed
-
- Carter, R.T. , Shaw, J.B. & Adams, R.A. (2014) Ontogeny of vocalization in Jamaican fruit bats with implications for the evolution of echolocation. Journal of Zoology, 293, 25–32.
-
- Currey, J.D. (2006) Bones: structure and mechanics. Princeton, NJ: Princeton University Press, pp. 124–129, 269.
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

