Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy
- PMID: 34927373
- PMCID: PMC8844476
- DOI: 10.1002/advs.202103444
Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy
Abstract
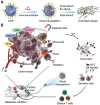
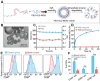
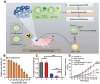
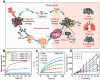
Cancer immunotherapy has achieved promising clinical progress over the recent years for its potential to treat metastatic tumors and inhibit their recurrences effectively. However, low patient response rates and dose-limiting toxicity remain as major dilemmas for immunotherapy. Stimuli-responsive nanoparticles (srNPs) combined with immunotherapy offer the possibility to amplify anti-tumor immune responses, where the weak acidity, high concentration of glutathione, overexpressions of enzymes, and reactive oxygen species, and external stimuli in tumors act as triggers for controlled drug release. This review highlights the design of srNPs based on tumor microenvironment and/or external stimuli to combine with different anti-tumor drugs, especially the immunoregulatory agents, which eventually realize synergistic immunotherapy of malignant primary or metastatic tumors and acquire a long-term immune memory to prevent tumor recurrence. The authors hope that this review can provide theoretical guidance for the construction and clinical transformation of smart srNPs for controlled drug delivery in synergistic cancer immunotherapy.
Keywords: clinical translation; controlled drug release; nanotechnology; smart nanocarrier; synergistic immunotherapy.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Goldberg M. S., Cell 2015, 161, 201. - PubMed
Publication types
MeSH terms
Grants and funding
- 51903050/National Natural Science Foundation of China
- 21874024/National Natural Science Foundation of China
- 22027805/National Natural Science Foundation of China
- 2019J01258/Natural Science Foundation of Fujian Province
- Opening Foundation of State Key Laboratory of Polymer Materials Engineering
- sklpme2019-4-34/Sichuan University
- 00221002/Key Program of Qingyuan Innovation Laboratory
- 2021T025/Fuzhou University Testing Fund of Precious Apparatus
- Opening Foundation of State Key Laboratory of Molecular Engineering of Polymers
- K2021-03/Fudan University
- 2020HZ06006/the Major Project of Science and Technology of Fujian Province
LinkOut - more resources
Full Text Sources
